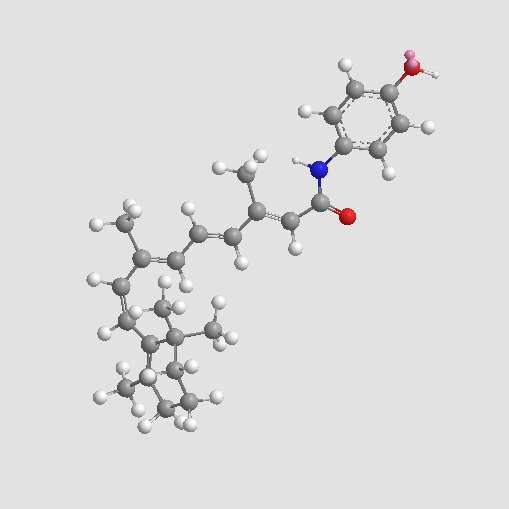
Fenretinide
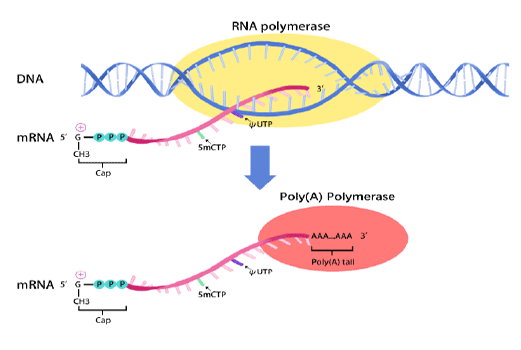
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
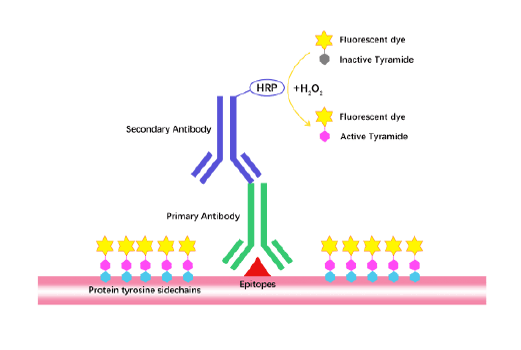
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
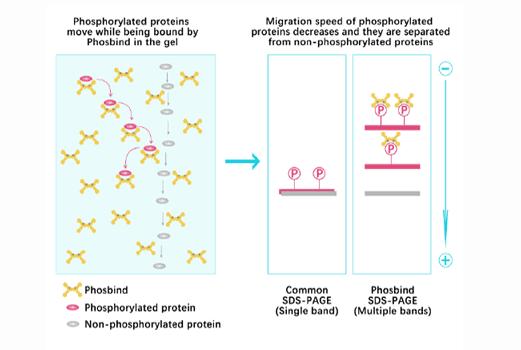
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
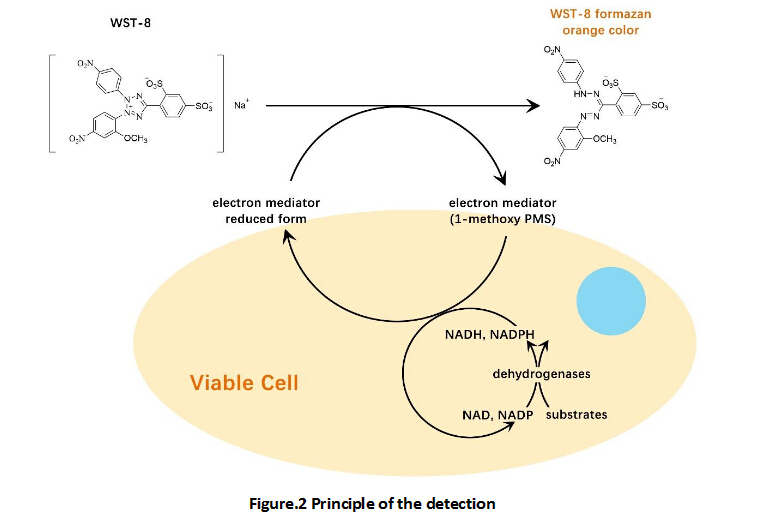
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
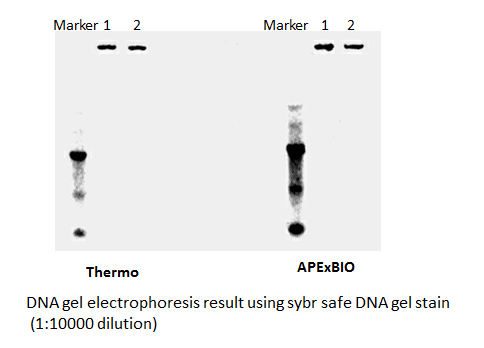
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
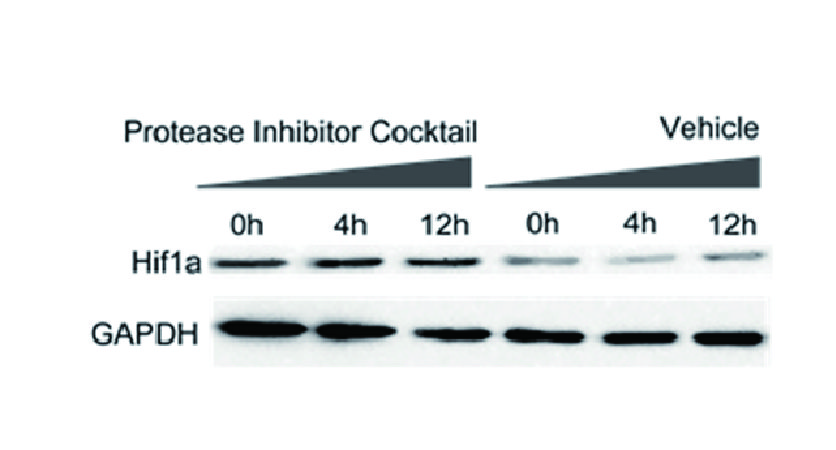
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Fenretinide(4HPR)是黏着斑激酶(FAK)的抑制剂[1]。
Fenretinide是一种维生素A类似物,能够抑制多种肿瘤细胞的生长,包括小细胞肺癌细胞、恶性造血细胞和乳腺癌细胞。Fenretinide也能保护妇女抵抗卵巢癌的发展。Fenretinide对于诸多妇科癌症细胞系起效,其中对两种卵巢癌细胞系(222和UCI 101)的IC50值仅为0.3和0.4 μM,对其它卵巢癌、宫颈癌与子宫内膜癌细胞系的IC50值为1-10 μM[2]。
Fenretinide能够诱导人前列腺癌细胞(HPC)凋亡。Fenretinide对LNCaP、DU145和PC-3的IC50值分别为0.9±0.16 μM、4.4±0.45 μM和3.0±1.0 μM。Fenretinide通过增加ROS和酶标的DNA断裂及梯状DNA形成来诱导细胞凋亡。并且,Fenretinide能通过干扰FAK/AKT/GSK3β通路和β-catenin的稳定性,来妨碍前列腺癌细胞的迁移和侵袭[1, 3]。
参考文献:
[1] Roberto Benelli, Stefano Monteghirfo, Roberta Venè, Francesca Tosettiand Nicoletta Ferrari. The chemopreventive retinoid 4HPR impairs prostate cancer cell migration and invasion by interfering with FAK/AKT/GSK3β pathway andβ-catenin stability. Molecular Cancer.2010, 9:142-154.
[2] Anita L. Sabichi, Denver T. Hendricks, Mary A. Bober, Michael J. Birrer. Retinoic acid receptorβexpression and growthinhibition of gynecologic cancer cells by thesynthetic retinoidn-(4-hydroxyphenyl) retinamide. Journal of the National Cancer Institute. 1998, 90(8): 597-605.
[3] Shi-Yong Sun, Ping Yue, and Reuben Lotan. Induction of apoptosis by n-(4-hydroxyphenyl)retinamide andits association with reactive oxygen species, nuclearretinoic acid receptors, and apoptosis-related genes in human prostate carcinoma cells.Molecular Pharmacology. 1999, 55:403–410.
- 1. Xiao-Han Tang, Marta Melis, et al. "Fenretinide Improves Intestinal Barrier Function and Mitigates Alcohol Liver Disease." Front Pharmacol. 2021 Mar 18;12:630557. PMID:33815111
- 2. Britt EL, Raman S, et al. "Combination of fenretinide and ABT-263 induces apoptosis through NOXA for head and neck squamous cell carcinoma treatment." PLoS One. 2019 Jul 5;14(7):e0219398. PMID:31276572
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 391.55 |
| Cas No. | 65646-68-6 |
| Formula | C26H33NO2 |
| Synonyms | 4-HPR; (4-Hydroxyphenyl)retinamide |
| Solubility | insoluble in H2O; ≥19.6 mg/mL in DMSO; ≥47.8 mg/mL in EtOH with gentle warming |
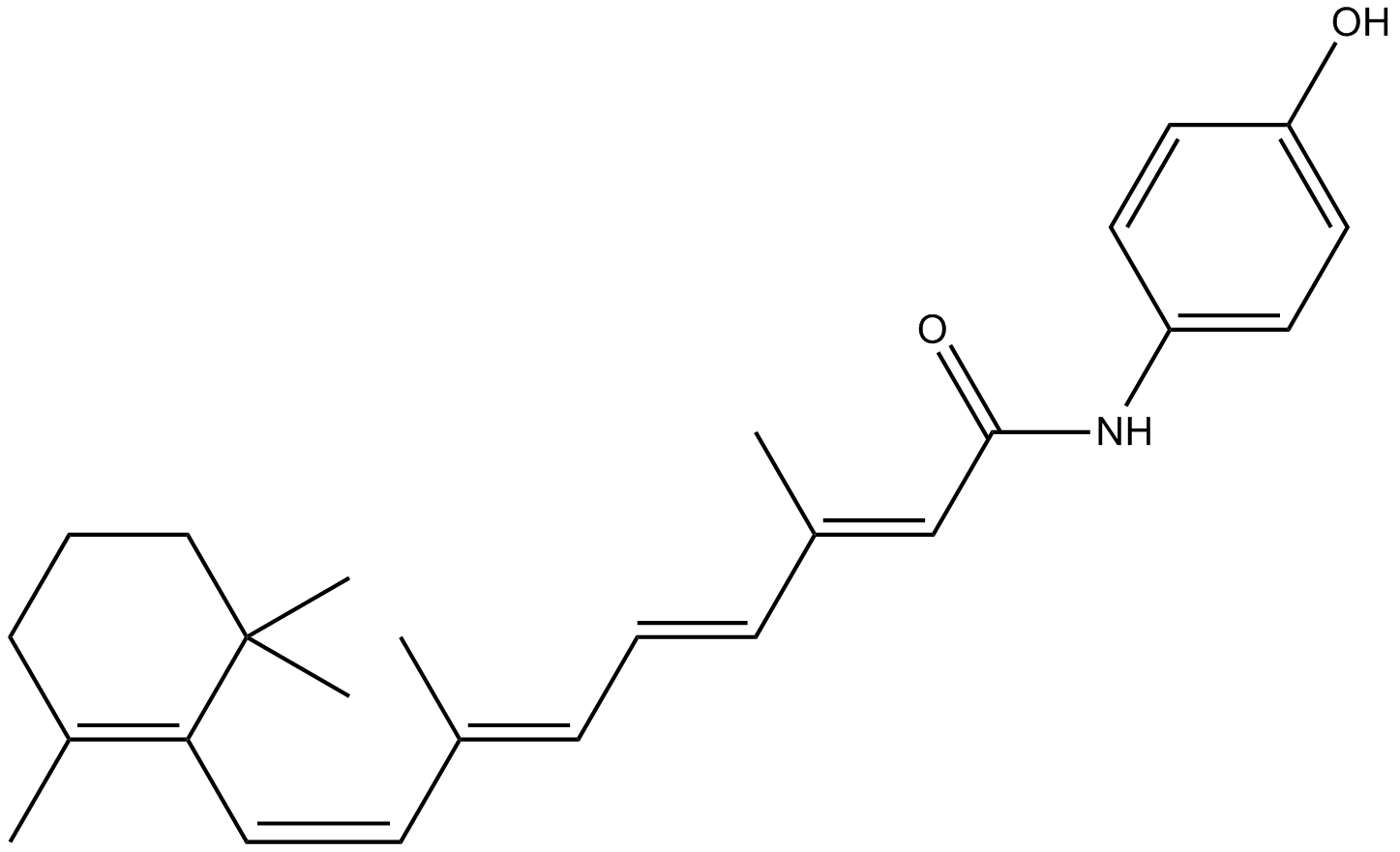
| Chemical Name | (2E,4E,6E,8E)-N-(4-hydroxyphenyl)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenamide |
| SDF | Download SDF |
| Canonical SMILES | CC1=C(C(CCC1)(C)C)C=CC(=CC=CC(=CC(=O)NC2=CC=C(C=C2)O)C)C |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 细胞实验 [1-3]: | |
|
细胞系 |
T-ALL细胞系,CCRF-CEM白血病细胞,CCRF-CEM和Jurkat细胞,OVCAR-5细胞系 |
|
溶解方法 |
该化合物在DMSO中的溶解度大于19.6 mg/mL。若获取更高浓度的溶液,可在37℃下孵育10分钟,随后在超声波浴中摇匀。-20℃以下可储存数月。 |
|
反应条件 |
>1 μM, 3 days |
|
应用 |
Fenretinide抑制许多肿瘤细胞的生长,包括小细胞肺癌、恶性造血细胞和乳腺癌细胞。在222和UCI 101卵巢癌细胞系中,Fenretinide的IC50值分别为0.3和0.4 μM。在选择的T-ALL细胞系中,Fenretinide显示出抗肿瘤活性。Fenretinide以剂量和时间依赖的方式抑制CCRF-CEM白血病细胞中的DES活性,导致内源性细胞dhCer含量的伴随增加。在CCRF-CEM和Jurkat细胞中,Fenretinide(3 μM)诱导dhCer积累。Fenretinide(>1 μM)抑制OVCAR-5细胞增殖和活力,在10 μM浓度下产生70-90%的生长抑制。Fenretinide(1 μM)预孵育3天后,显著抑制OVCAR-5侵袭。 |
| 动物实验[4,5]: | |
|
动物模型 |
HFD喂养的雄性C57Bl/6小鼠,NOD/SCID小鼠 |
|
给药剂量 |
腹腔注射,10 mg/kg |
|
应用 |
在HFD喂养的雄性C57Bl/6小鼠中,Fenretinide (10 mg/kg, i.p.)选择性地抑制神经酰胺积聚。Fenretinide改善葡萄糖耐量和胰岛素敏感性。在NOD/SCID小鼠中,向Fenretinide中添加25 mg/kg酮康唑增加了4-HPR血浆水平。 |
|
注意事项 |
由于实验环境的不同,实际溶解度可能与理论值略有不同,请测试室内所有化合物的溶解度。 |
|
References: [1]. Apraiz, Aintzane., et al. Dihydroceramide accumulation and reactive oxygen species are distinct and nonessential events in 4-HPR-mediated leukemia cell death. Biochemistry and Cell Biology (2012), 90(2), 209-223. [2]. Golubkov V, et al. Action of fenretinide (4-HPR) on ovarian cancer and endothelial cells. Anticancer Res. 2005 Jan-Feb;25(1A):249-53. [3]. Anita L. Sabichi, Denver T. Hendricks, Mary A. Bober, Michael J. Birrer. Retinoic acid receptorβexpression and growthinhibition of gynecologic cancer cells by thesynthetic retinoidn-(4-hydroxyphenyl) retinamide. Journal of the National Cancer Institute. 1998, 90(8): 597-605. [4]. Bikman, Benjamin T., et al. Fenretinide Prevents Lipid-induced Insulin Resistance by Blocking Ceramide Biosynthesis. Journal of Biological Chemistry (2012), 287(21), 17426-17437. [5]. Cooper JP, et al. Fenretinide metabolism in humans and mice: utilizing pharmacological modulation of its metabolic pathway to increase systemic exposure. Br J Pharmacol. 2011 Jul;163(6):1263-75. |
|
| Description | Fenretinide是人工合成的维甲酸衍生物。 | |||||
| 靶点 | RAR | |||||
| IC50 | ||||||
质量控制和MSDS
- 批次:
化学结构
相关生物数据

相关生物数据