Cyanine dyes
Cyanine dyes are molecules containing polymethine bridges between two nitrogen atoms with a delocalized charge which have been used for many years as dyes in life science. Since the Cy3, Cy5 and Cy7 were made commercially available as succinimidyl esters in the early 1990s, cyanine dyes began to be used widely as labels for nucleic acids. Because of the good properties of low non-specific binding to biomolecules and bright fluorescence owing to their huge extinction coefficients and good quantum yields, the Cy dyes have been widespread used in DNA and RNA labelling. Cyanine dyes can be divided into two groups based on water solubility: non-sulfonated cyanines and sulfonated cyanines.
charge which have been used for many years as dyes in life science. Since the Cy3, Cy5 and Cy7 were made commercially available as succinimidyl esters in the early 1990s, cyanine dyes began to be used widely as labels for nucleic acids. Because of the good properties of low non-specific binding to biomolecules and bright fluorescence owing to their huge extinction coefficients and good quantum yields, the Cy dyes have been widespread used in DNA and RNA labelling. Cyanine dyes can be divided into two groups based on water solubility: non-sulfonated cyanines and sulfonated cyanines.
Non-sulfonated cyanines
Available non-sulfonated dyes include Cy3, Cy3.5, Cy5, Cy5.5, Cy7, and Cy7.5. Cy stands for 'cyanine', and the first digit identifies the number of carbon atoms between the indolenine groups. The suffix .5 is added for benzo-fused cyanines. Most derivatives of non-sulfonated cyanines have low aqueous solubility except for hydrochlorides of hydrazides and amines. They are organic co-solvent soluble (5-20% of DMF or DMSO). When these molecules are used for biomolecule labeling, they should be dissolved in organic solvent first, and added to a solution of biomolecule (protein, peptide, amino-labeled DNA) in appropriate aqueous buffer. Fluorescent properties of non-sulfonated cyanines have little dependence on solvent and surrounding.
Sulfonated cyanines
Available sulfonated cyanines include sulfo-Cy3, sulfo-Cy5, and sulfo-Cy7, which have additional sulfo-groups that facilitate dissolution of dye molecules in aqueous phase. Charged sulfonate groups decrease aggregation of dye molecules and heavily labeled conjugates. Sulfonated cyanines are highly water soluble, and they do not use organic co-solvent for the labeling in aqueous environment.
-
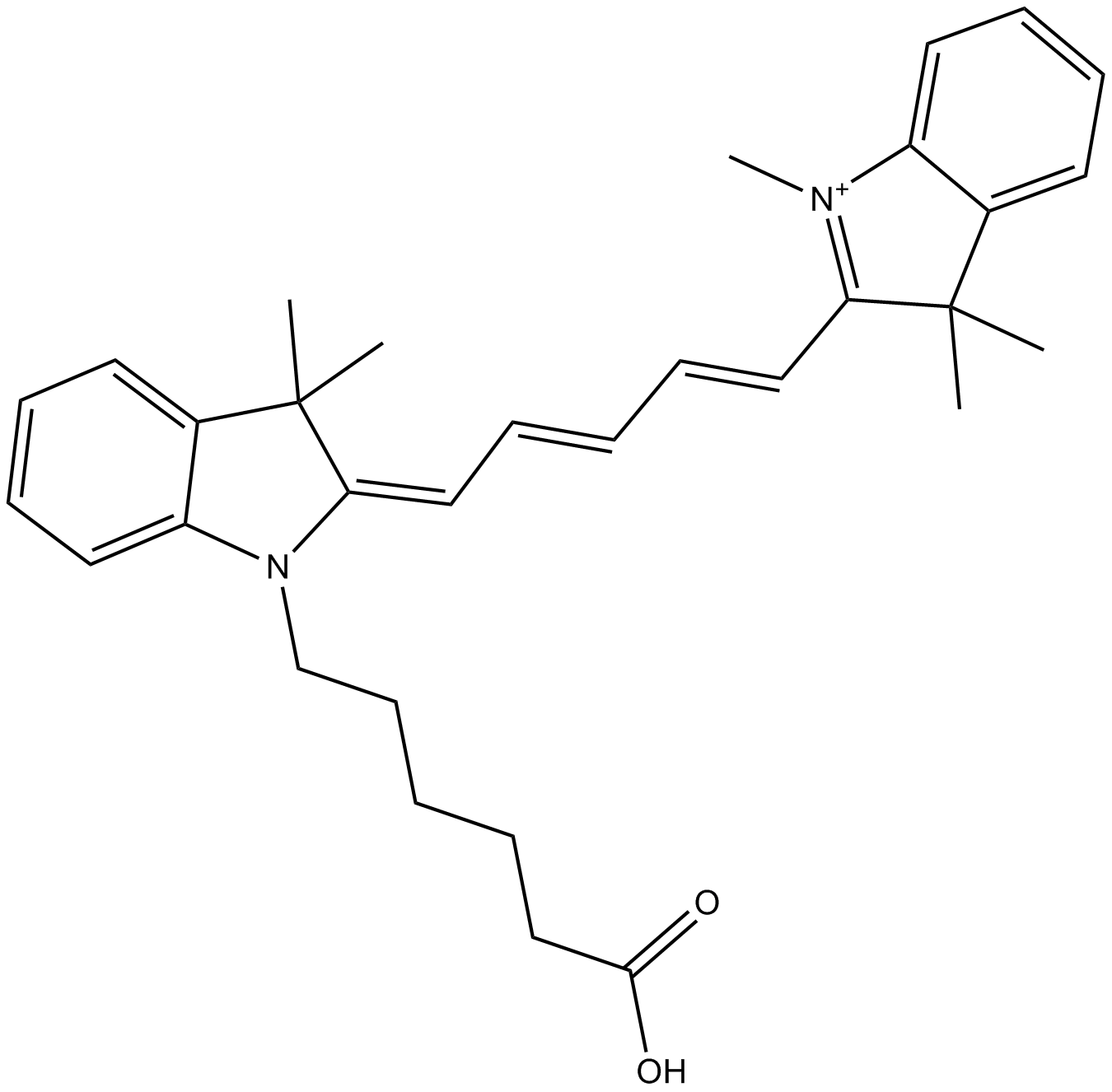 A8133 Cy5 carboxylic acid (non-sulfonated)Summary: 非反应性荧光团,用于对照实验和校准
A8133 Cy5 carboxylic acid (non-sulfonated)Summary: 非反应性荧光团,用于对照实验和校准 -
 A8132 Cy3 carboxylic acid (non-sulfonated)Summary: 非反应性荧光团,用于对照实验和校准
A8132 Cy3 carboxylic acid (non-sulfonated)Summary: 非反应性荧光团,用于对照实验和校准 -
 A8116 Cy7.5 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸
A8116 Cy7.5 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸 -
 A8115 Cy7 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸
A8115 Cy7 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸 -
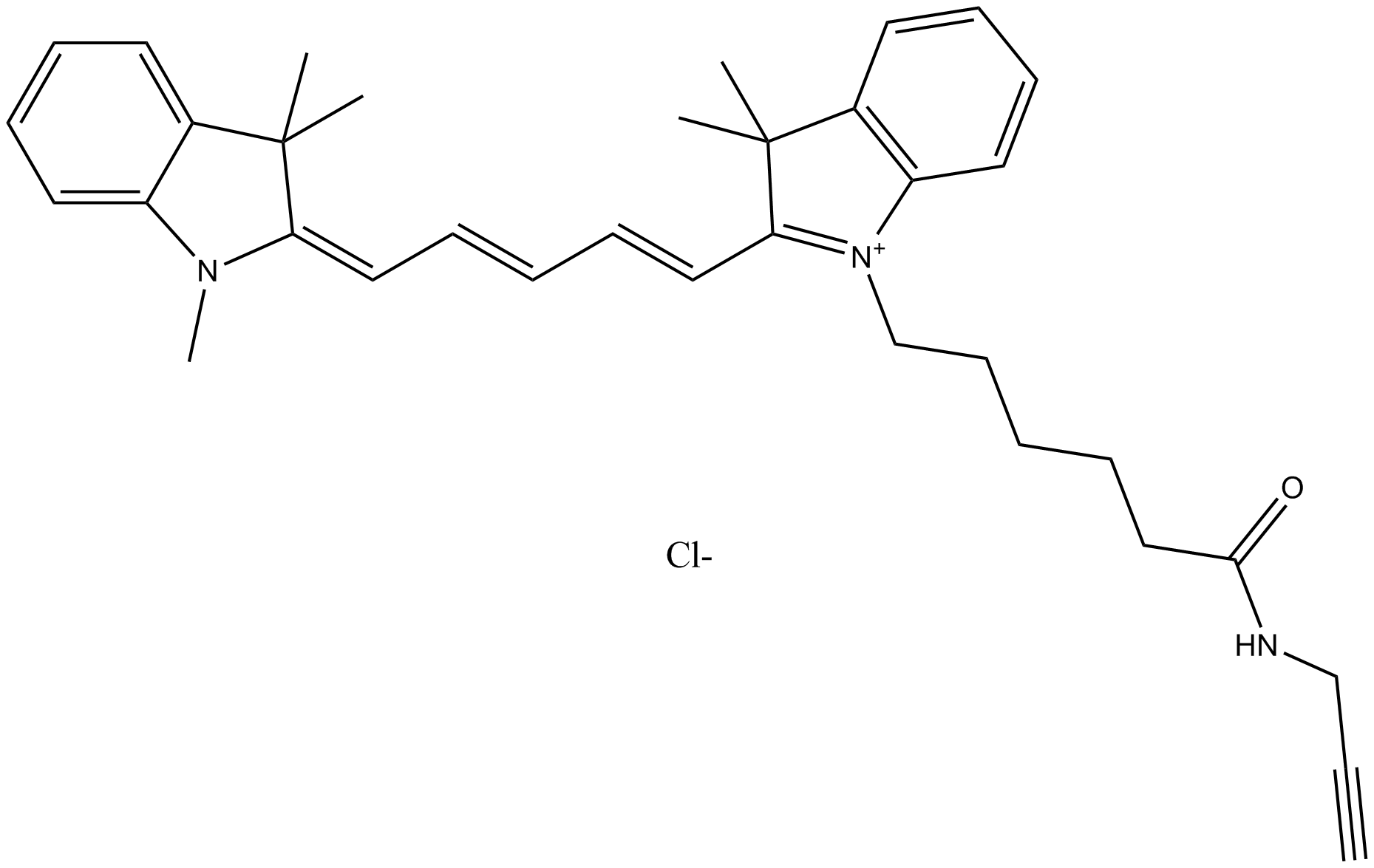 A8131 Cy5 alkyne (non-sulfonated)Summary: 用于点击化学,标记任何带有叠氮基的分子
A8131 Cy5 alkyne (non-sulfonated)Summary: 用于点击化学,标记任何带有叠氮基的分子 -
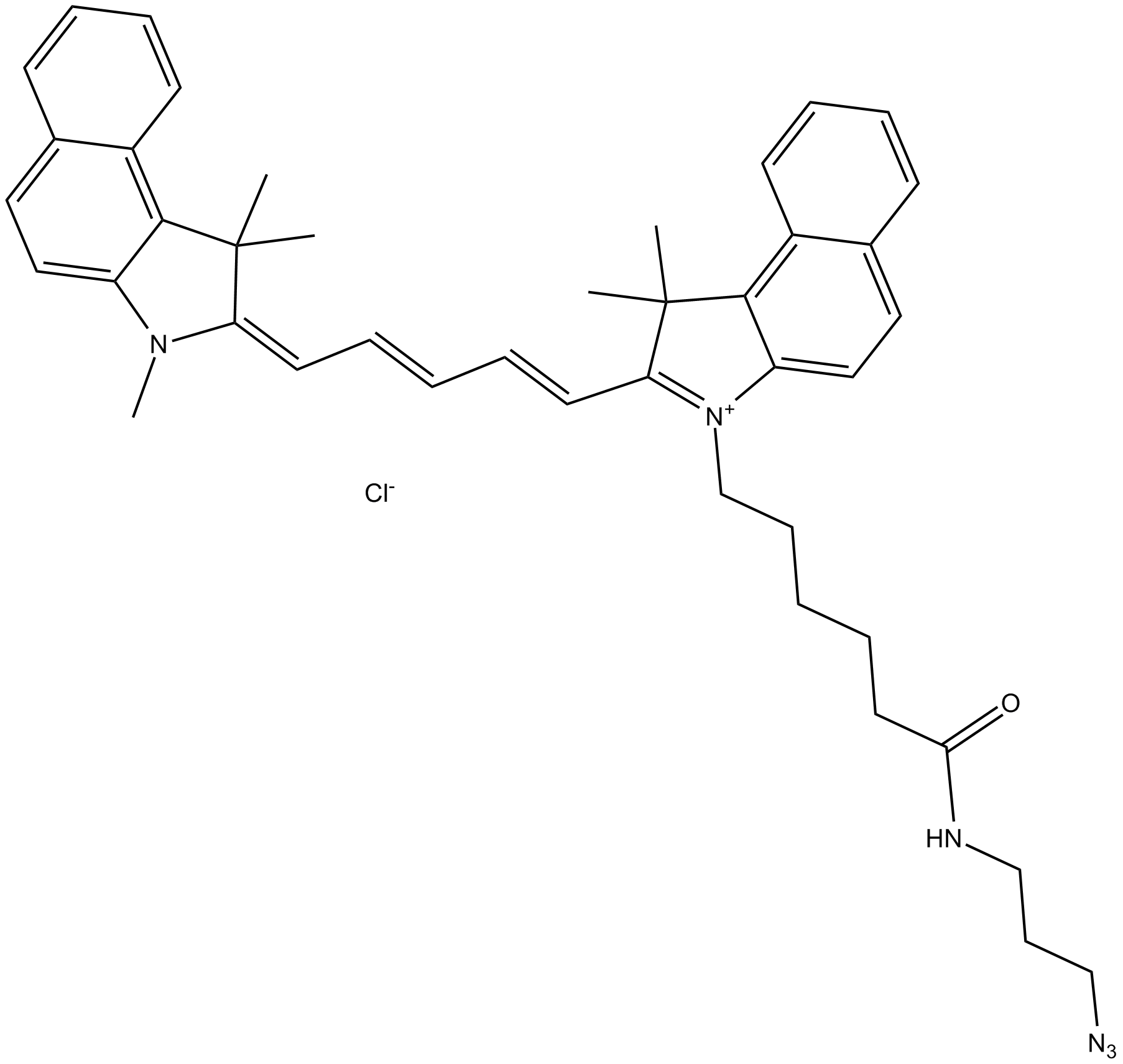 A8114 Cy5.5 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸
A8114 Cy5.5 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸 -
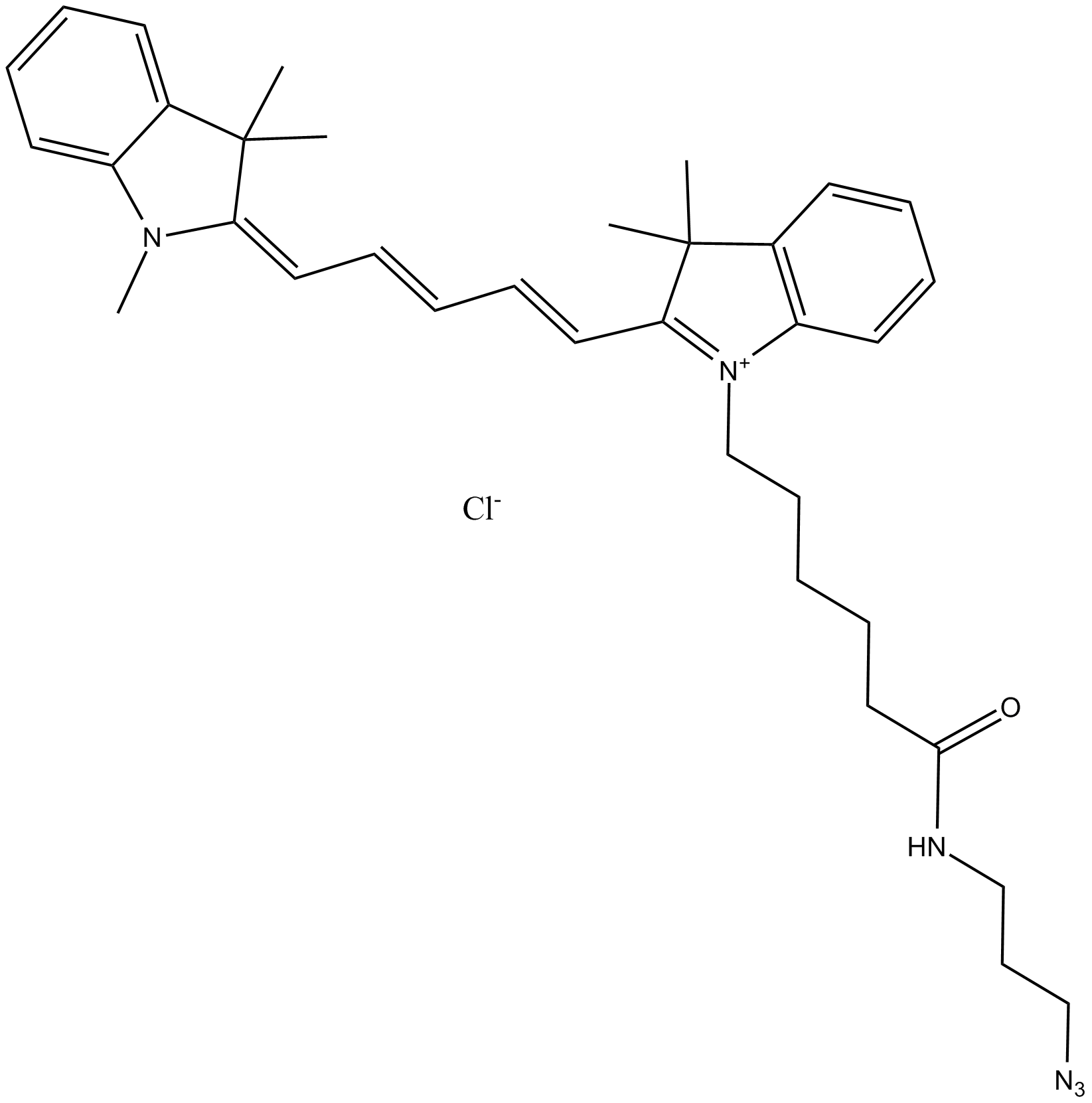 A8113 Cy5 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸
A8113 Cy5 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸 -
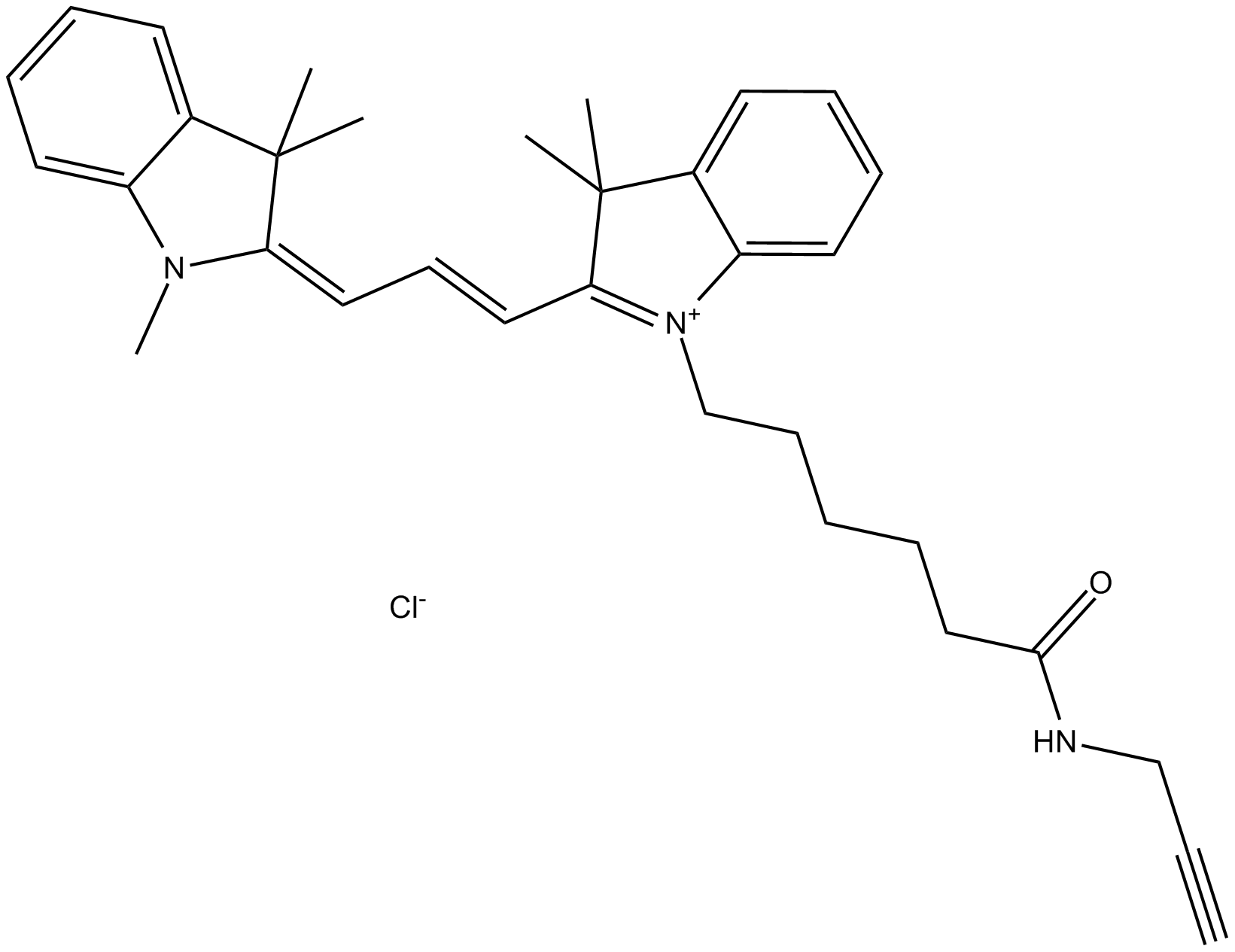 A8130 Cy3 alkyne (non-sulfonated)Summary: 用于点击化学,标记任何带有叠氮基的分子
A8130 Cy3 alkyne (non-sulfonated)Summary: 用于点击化学,标记任何带有叠氮基的分子 -
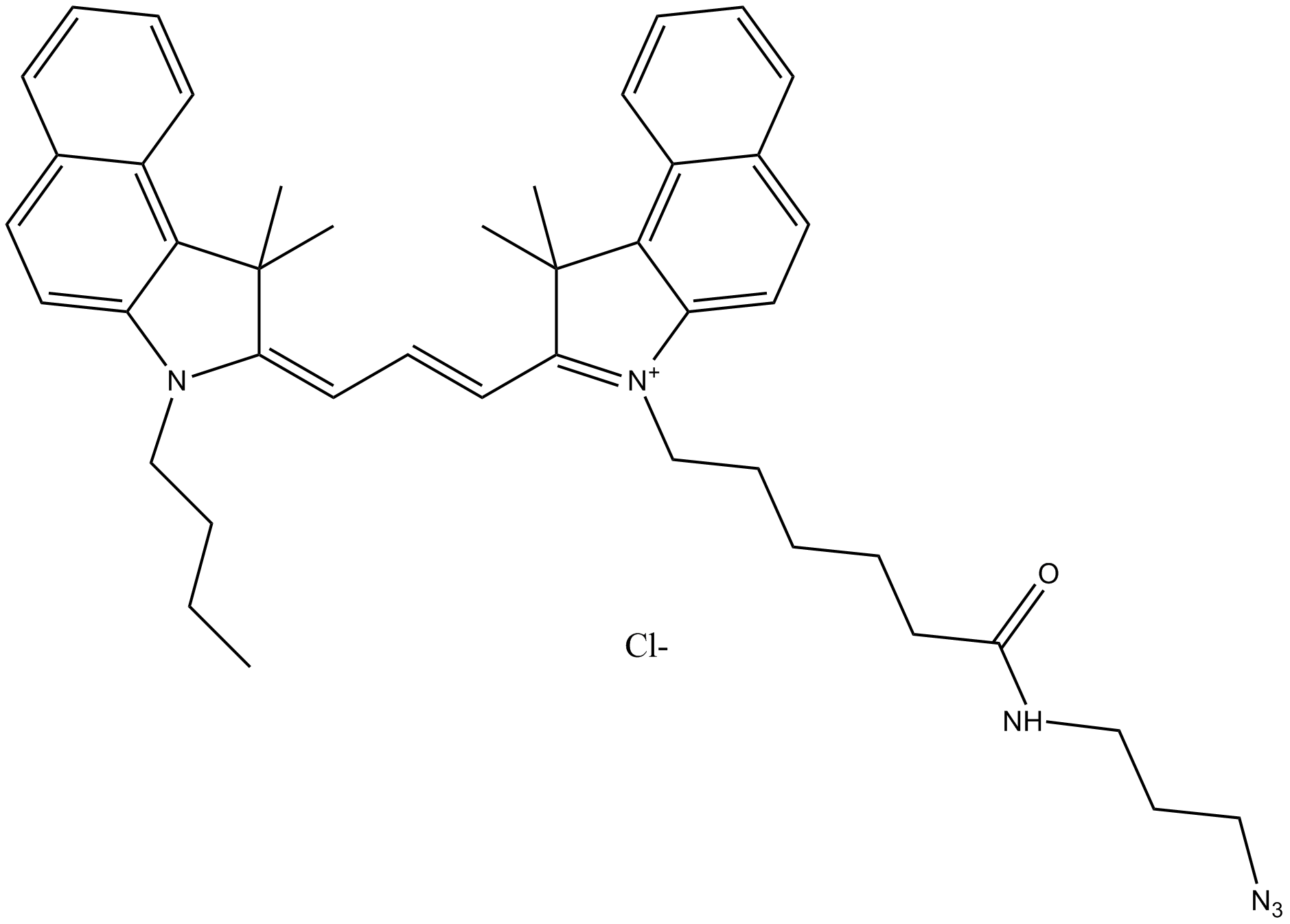 A8112 Cy3.5 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸
A8112 Cy3.5 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸 -
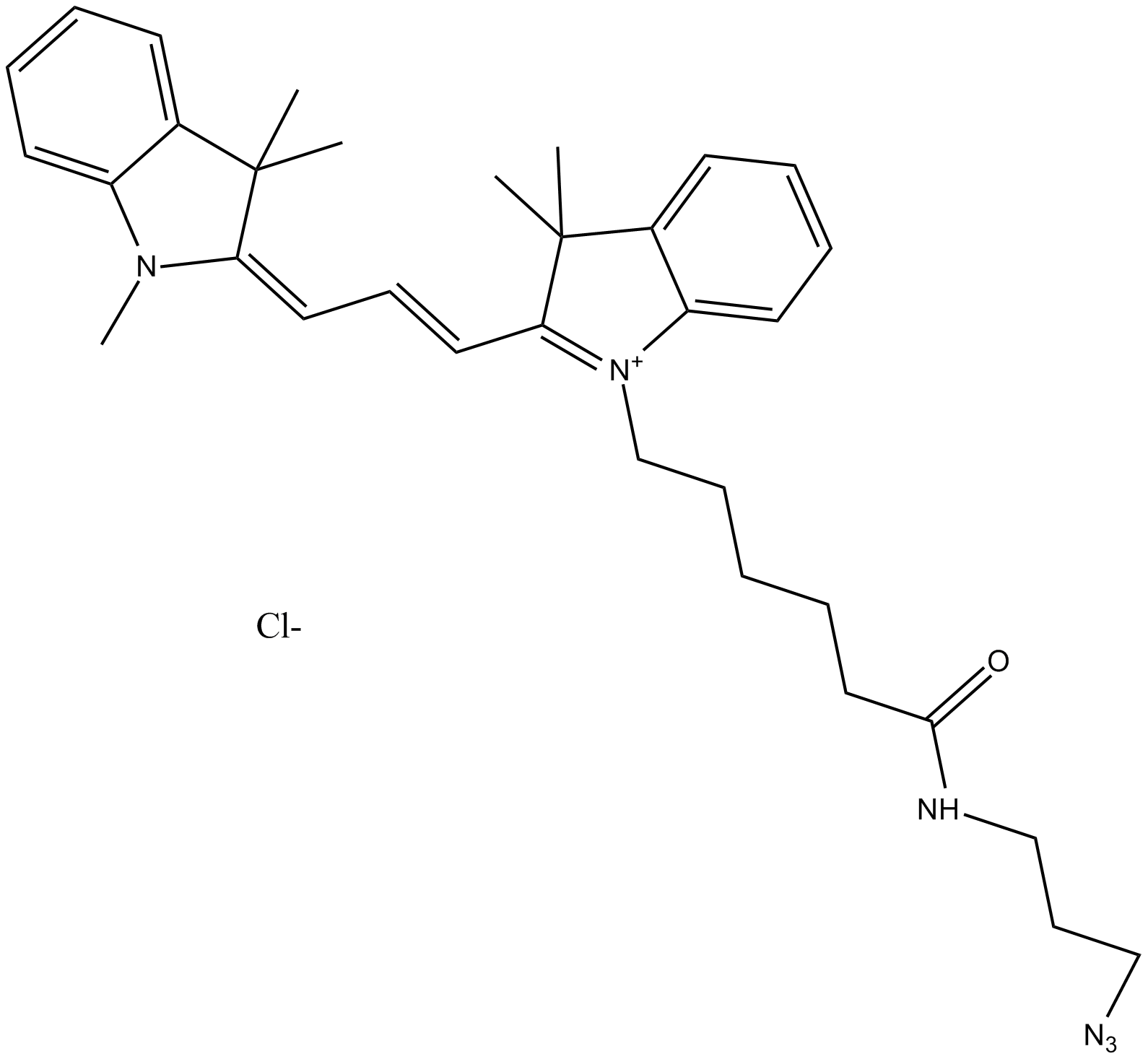 A8111 Cy3 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸
A8111 Cy3 azide (non-sulfonated)Summary: 用于点击化学,标记炔烃修饰的寡核苷酸

