DUB
Deubiquitinating enzymes (DUBs), belonging to the superfamily of proteases, are a group of enzymes that catalyze the cleavage of ubiquitin from ubiquitin-linked molecules after the terminal carbonyl of the last residue of ubiquitin (Gly76). Based on the mechanism of catalysis, DUBs is divided into two classes, cysteine proteases, which interact with substrates through the thiol group of a cysteine in the active site, and metalloproteases, which form a noncovalent intermediate with the substrate through a Zn2+ bound polarized water molecule. The cysteine protease DUBs have different Ub-protease domains and hence are further divided into four subclasses, ubiquitin-specific protease (USP), ubiquitin C-terminal hydrolase (UCH), Otubain protease (OTU) and Machado-Joseph disease protease (MJD); while all metalloprotease DUBs have one Ub protease domain called JAMM (JAB1/MPN/Mov34 metalloenzyme).
-
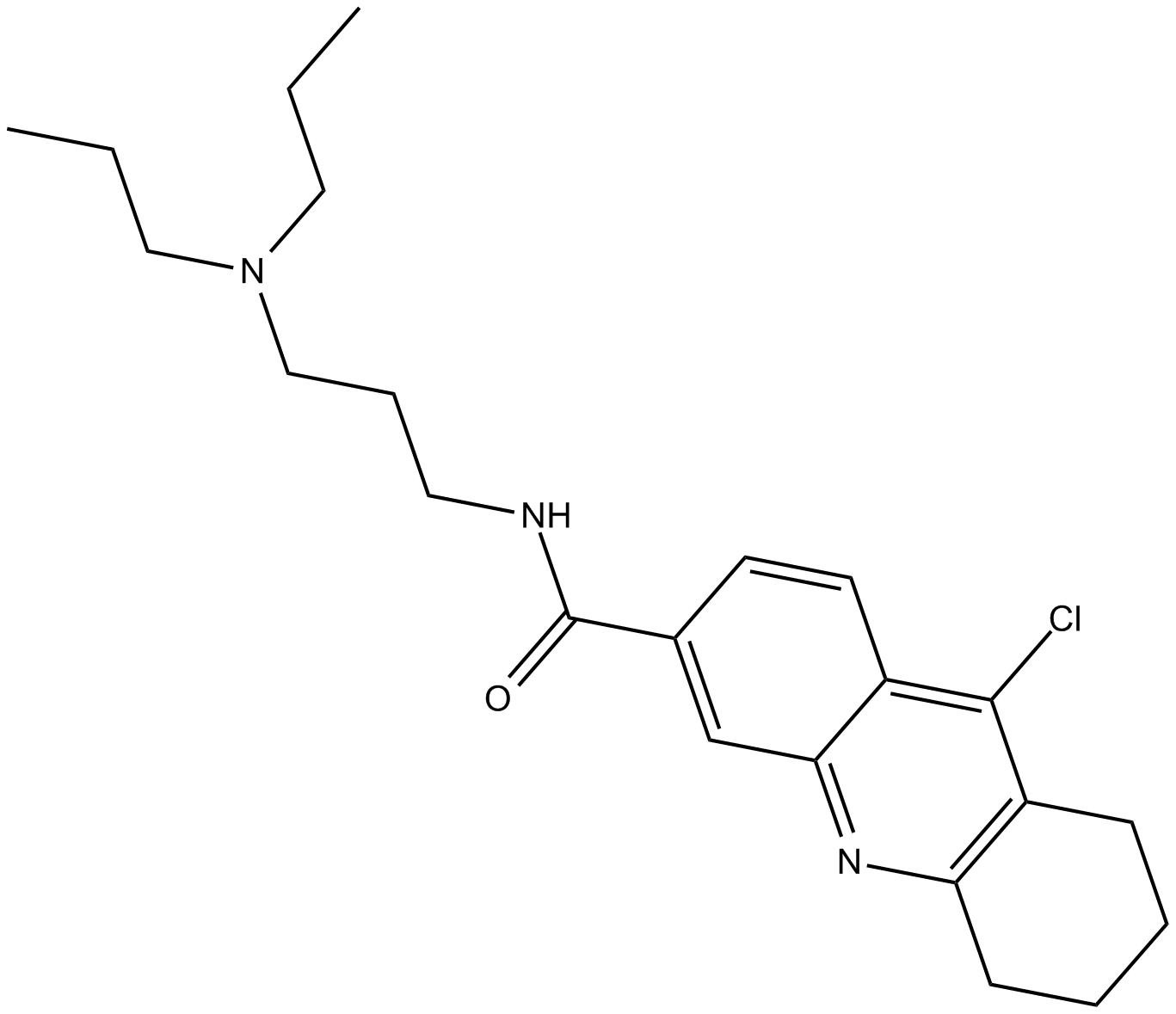 A8740 C598-0466Summary: USP7抑制剂
A8740 C598-0466Summary: USP7抑制剂 -
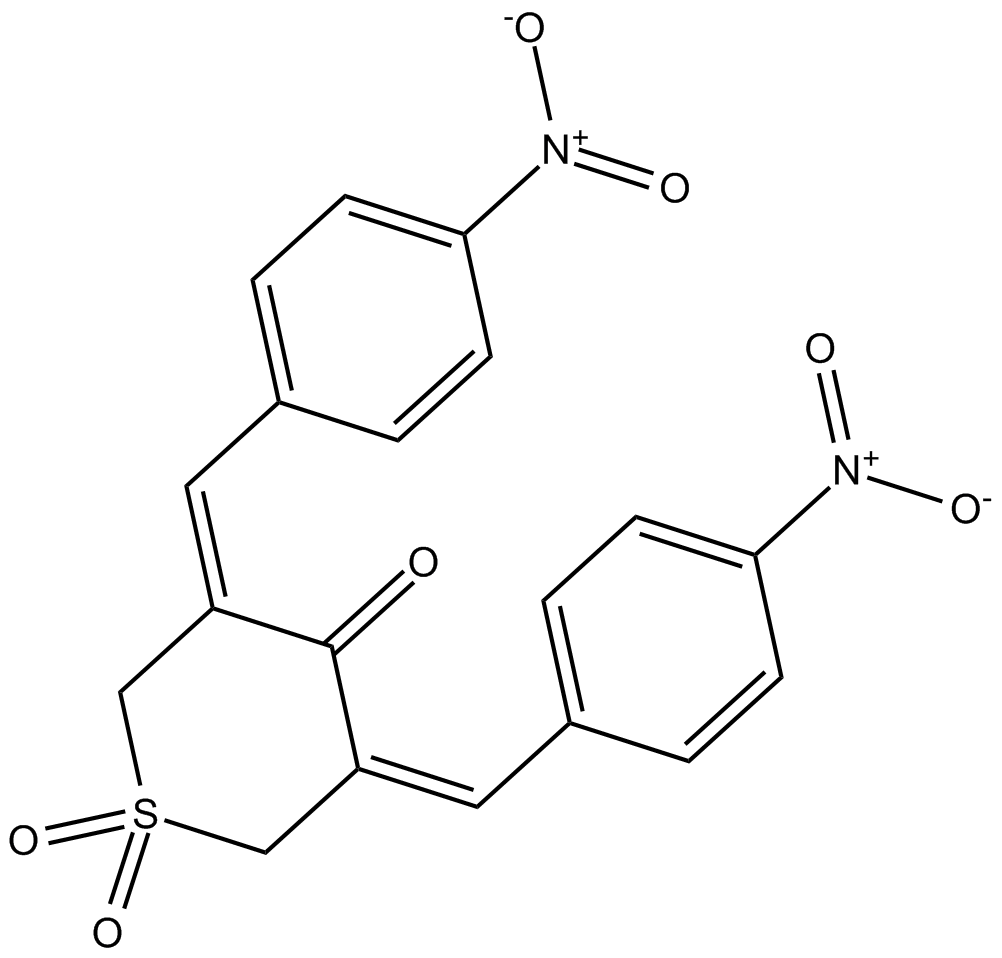 C3250 Ubiquitin Isopeptidase Inhibitor ISummary: 泛素异肽酶抑制剂
C3250 Ubiquitin Isopeptidase Inhibitor ISummary: 泛素异肽酶抑制剂 -
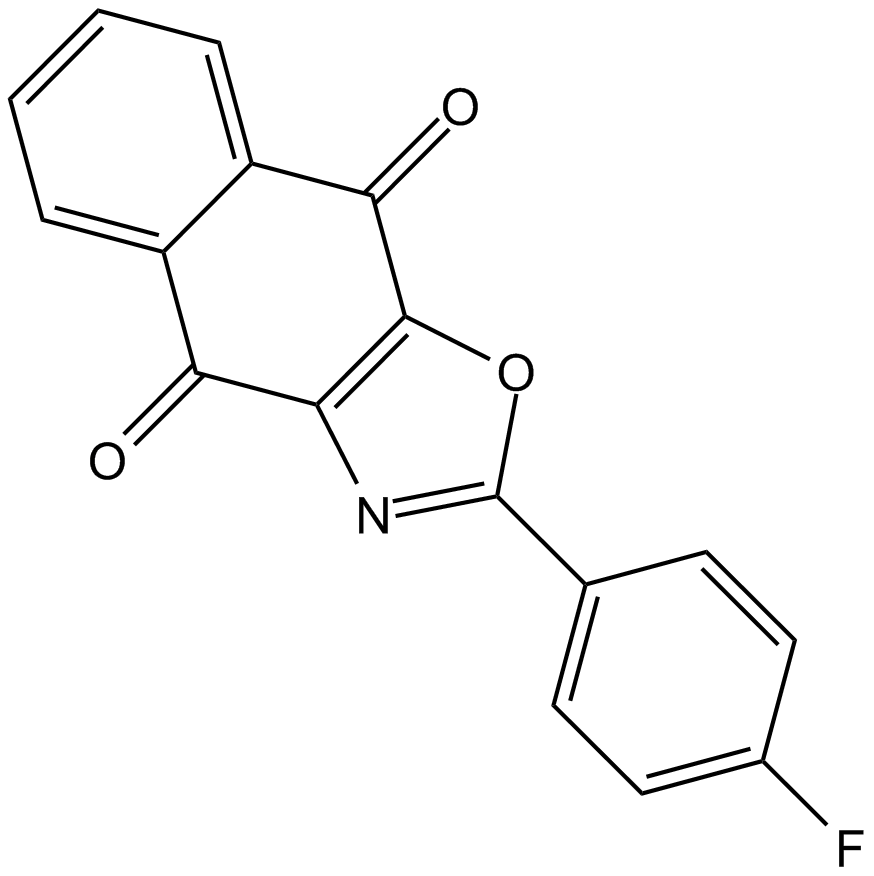 A8693 C527Target: USP1/USF1 complexSummary: USP1/USF1复合体抑制剂
A8693 C527Target: USP1/USF1 complexSummary: USP1/USF1复合体抑制剂 -
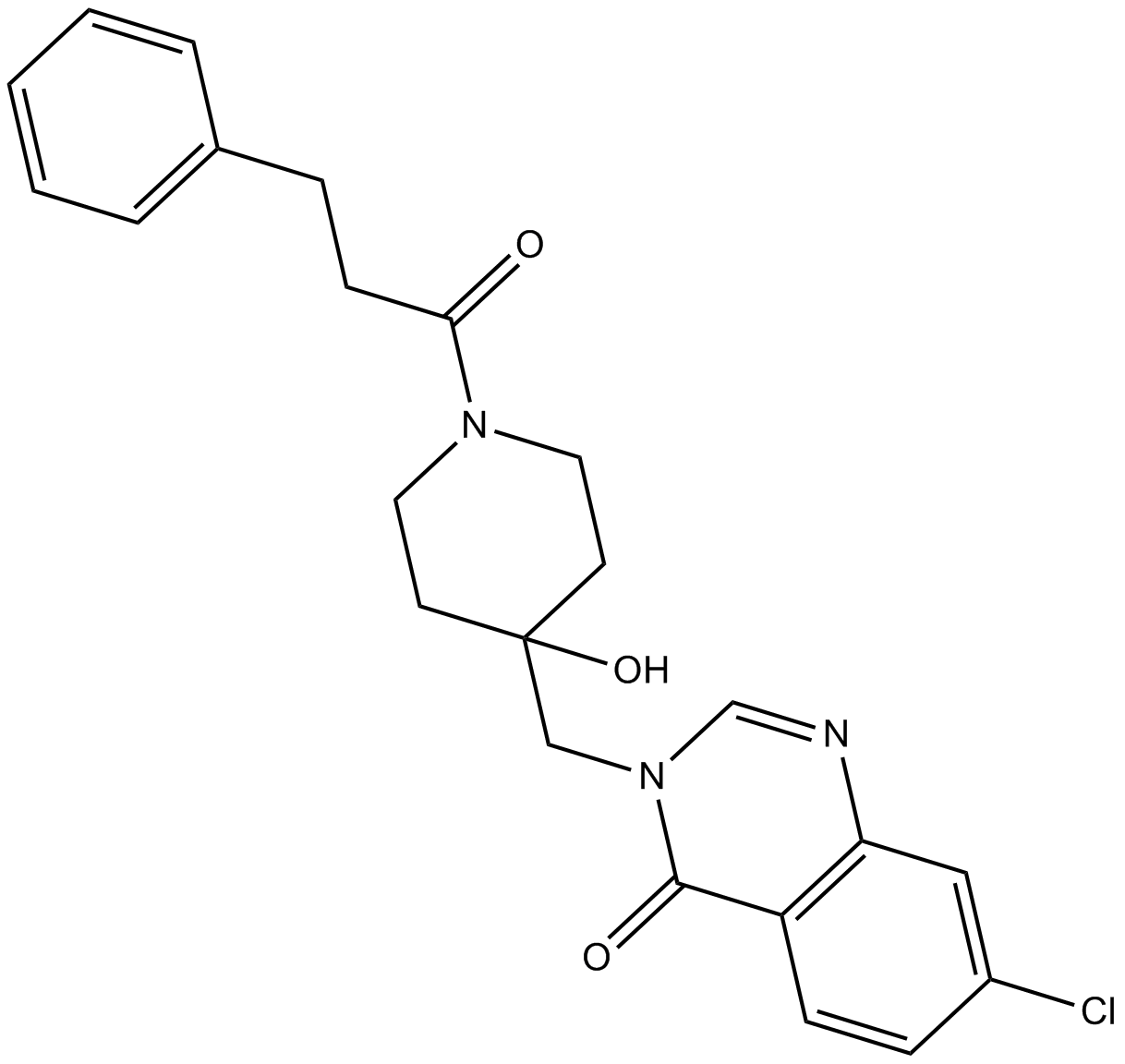 B4841 USP7-IN-1
B4841 USP7-IN-1 -
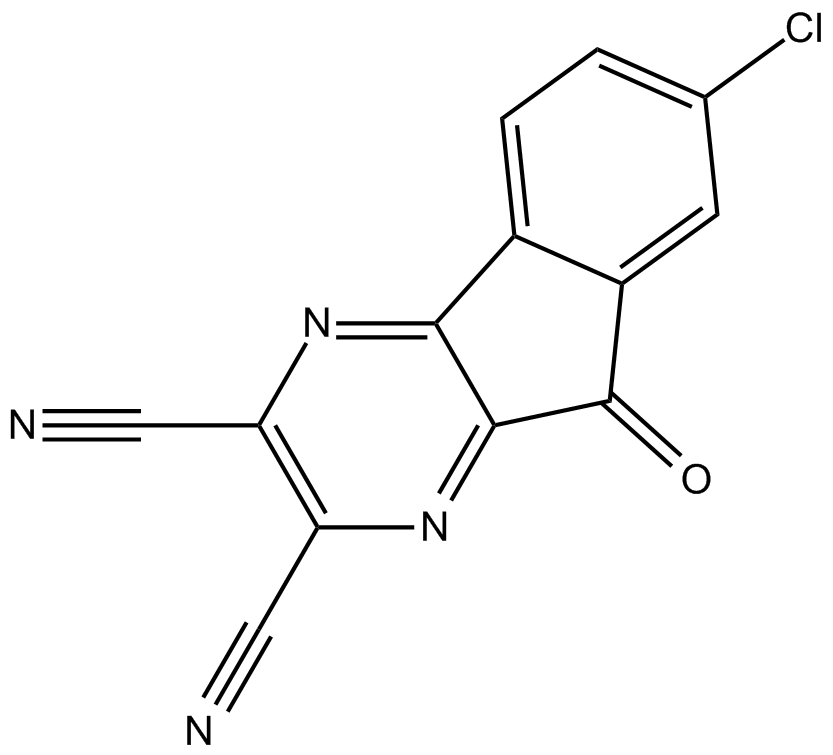 B5550 HBX 41108Summary: 泛素特异性蛋白酶(USP)7抑制剂
B5550 HBX 41108Summary: 泛素特异性蛋白酶(USP)7抑制剂 -
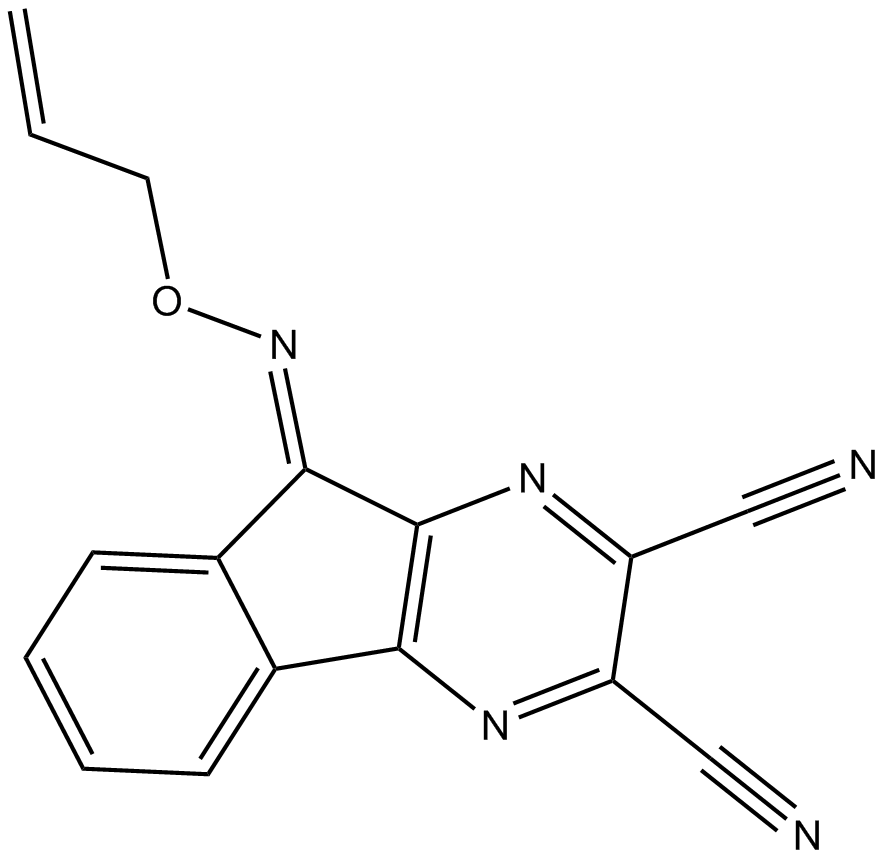 B3516 DUBs-IN-3Summary: 有效的去泛素化酶抑制剂
B3516 DUBs-IN-3Summary: 有效的去泛素化酶抑制剂 -
 B3515 DUBs-IN-2Summary: 去泛素化酶抑制剂
B3515 DUBs-IN-2Summary: 去泛素化酶抑制剂 -
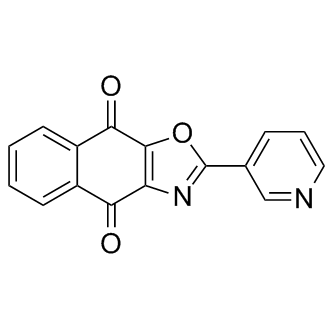 B1307 SJB3-019ATarget: USPSummary: Usp1抑制剂
B1307 SJB3-019ATarget: USPSummary: Usp1抑制剂 -
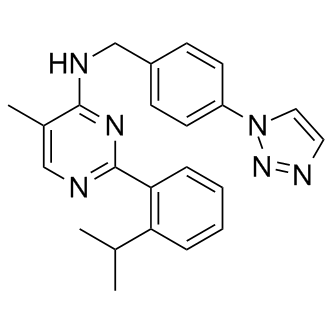 B1317 ML-323Target: Deubiquitinating enzymes (DUBs)Summary: USP1-UAF1抑制剂
B1317 ML-323Target: Deubiquitinating enzymes (DUBs)Summary: USP1-UAF1抑制剂 -
 B1097 HBX 19818
B1097 HBX 19818

