Tyrosine Kinase

Receptor tyrosine kinases bind to extracellular ligands/growth factors, which promotes receptor dimerization and autophosphorylation of receptor tyrosine residues. This triggers a cascade of downstream events through phosphorylation of intracellular proteins that ultimately transduce the extracellular signal to the nucleus, causing changes in gene expression. Receptor tyrosine kinases include EGFR/ErbB, PDGFR, VEGFR, FGFR and MET subfamilies etc. Dysfunctions in tyrosine phosphorylation are linked to oncogenic transformation. In additions, various adaptor and effector proteins couple to carboxy-terminal of an active kinase. For instance, binding of the GRB2 adaptor protein activates EGFR and MAPK/ERK signaling.
Non-receptor tyrosine kinases involve many well-defined proteins (e.g. the Src family kinases, c-Abl, and Jak kinases) and other kinases which regulates cell growth and differentiation. For example, Src family kinases are curial for activating and inhibitory pathways in the innate immune response.
-
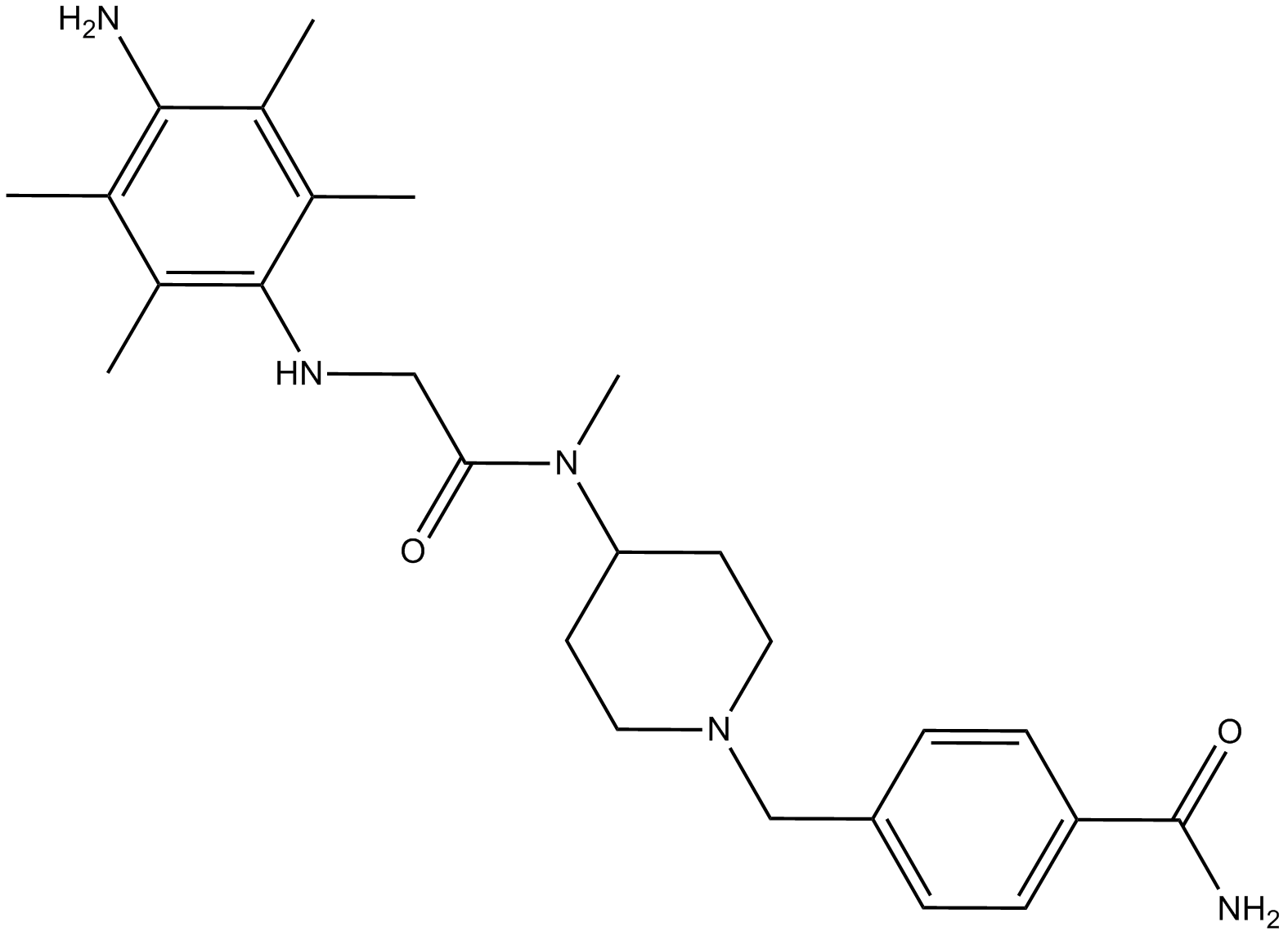 B8533 SUN11602Summary: 一种新型的碱性苯胺化合物,具有成纤维细胞生长因子活性
B8533 SUN11602Summary: 一种新型的碱性苯胺化合物,具有成纤维细胞生长因子活性 -
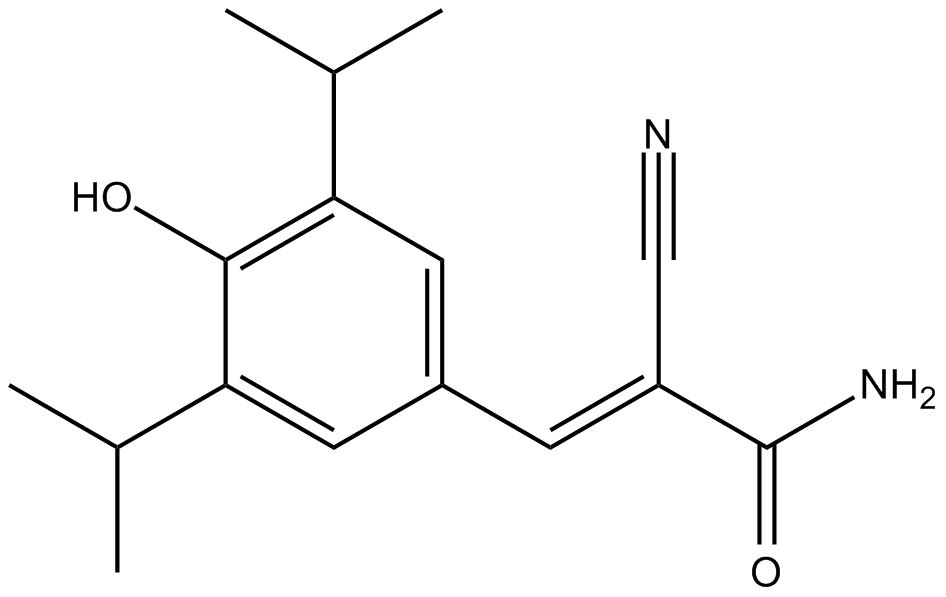 B8517 ST271Summary: 有效的蛋白酪氨酸激酶(PTK)抑制剂
B8517 ST271Summary: 有效的蛋白酪氨酸激酶(PTK)抑制剂 -
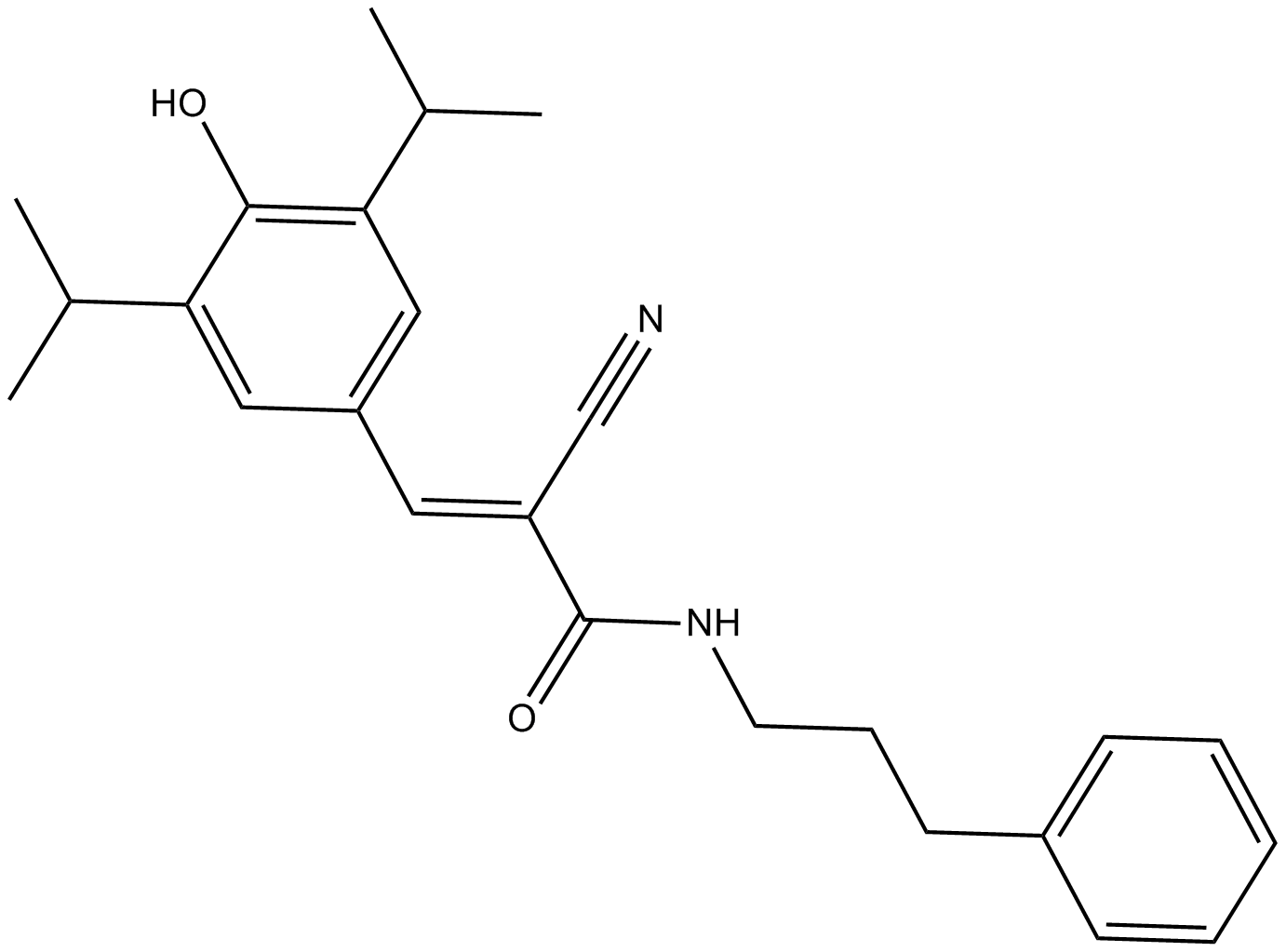 B8516 SU1498Summary: 选择性的 VEGFR2 抑制剂
B8516 SU1498Summary: 选择性的 VEGFR2 抑制剂 -
 B6054 EAI045Summary: L858R/T790M EGFR突变的抑制剂
B6054 EAI045Summary: L858R/T790M EGFR突变的抑制剂 -
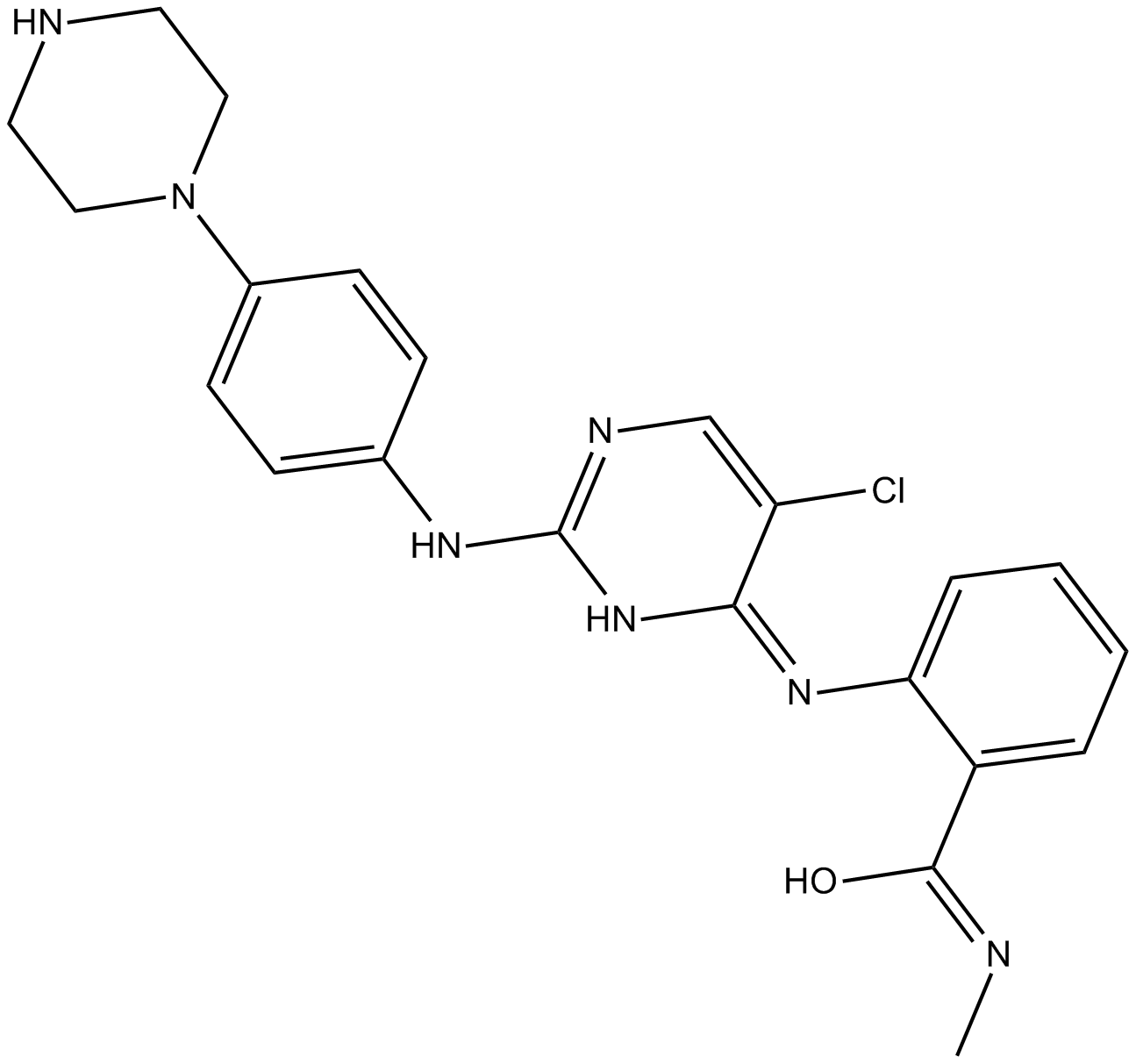 B5843 CTX0294885Target: KinasesSummary: Pan-激酶抑制剂
B5843 CTX0294885Target: KinasesSummary: Pan-激酶抑制剂 -
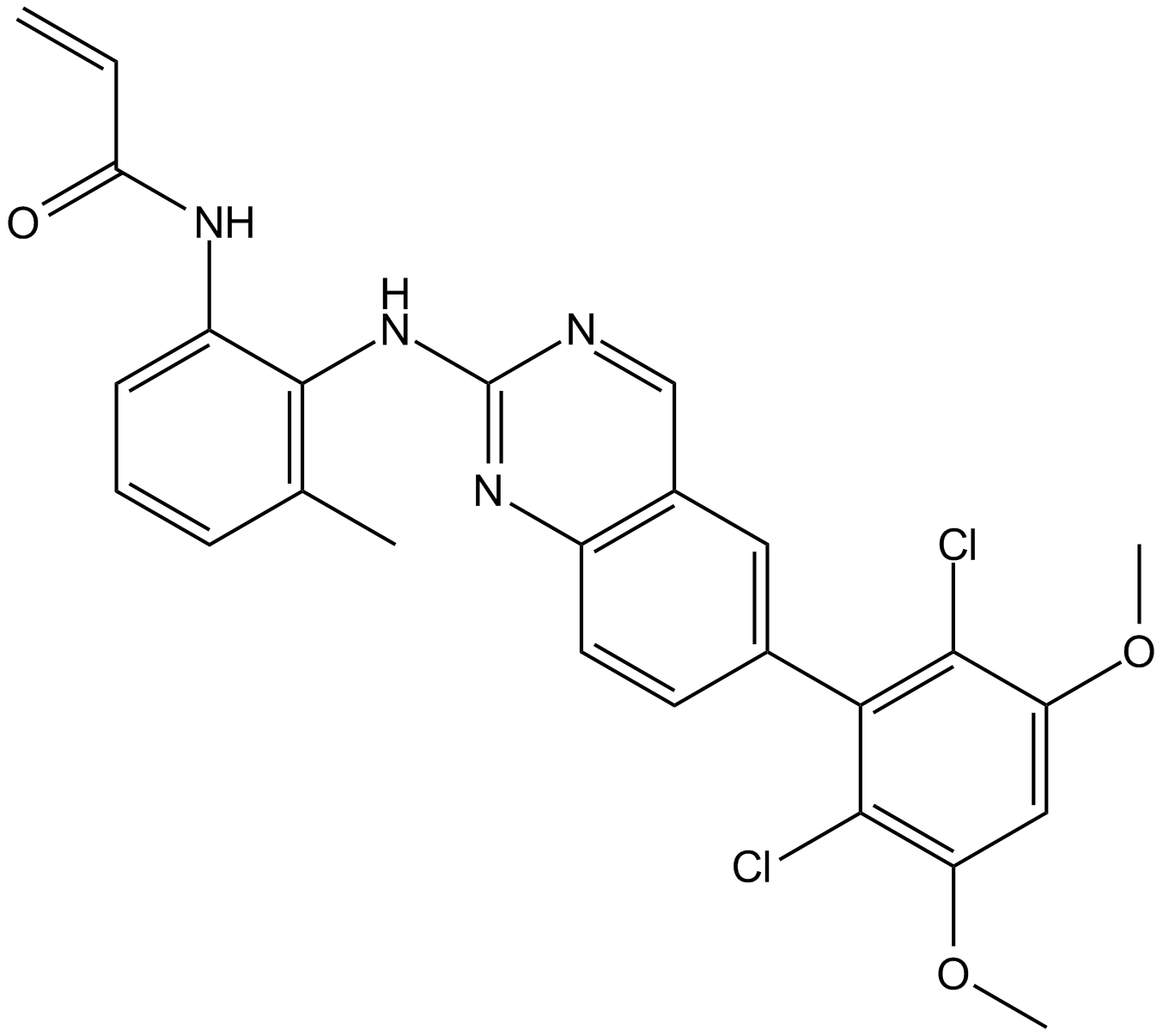 A8706 BLU9931Target: FGFRSummary: 有效的FGFR4不可逆抑制剂
A8706 BLU9931Target: FGFRSummary: 有效的FGFR4不可逆抑制剂 -
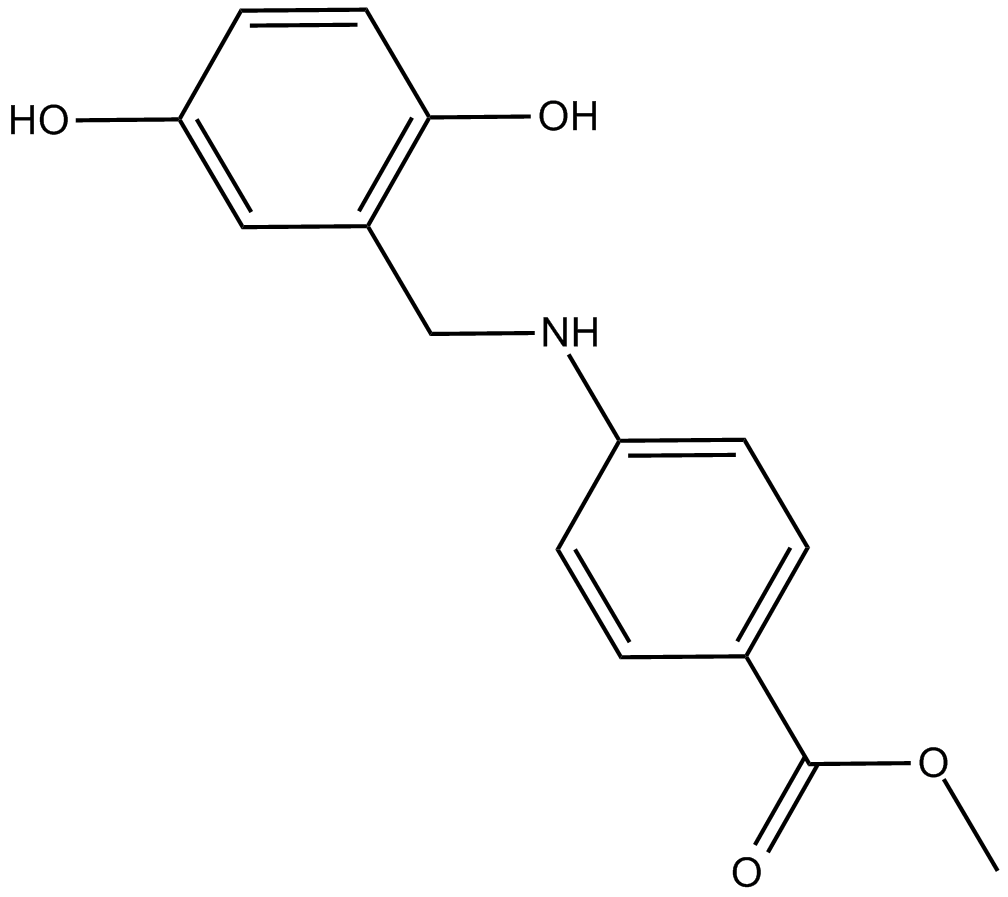 C5826 AG957Summary: 酪氨酸磷酸化抑制剂
C5826 AG957Summary: 酪氨酸磷酸化抑制剂 -
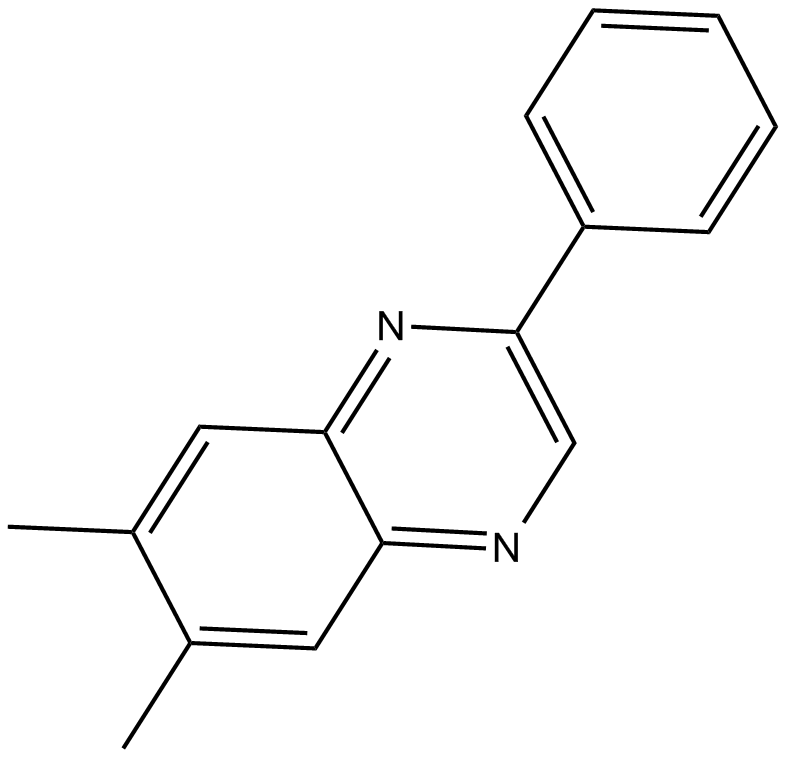 C5465 AG-1295Summary: PDGF受体激酶的有效选择性抑制剂
C5465 AG-1295Summary: PDGF受体激酶的有效选择性抑制剂 -
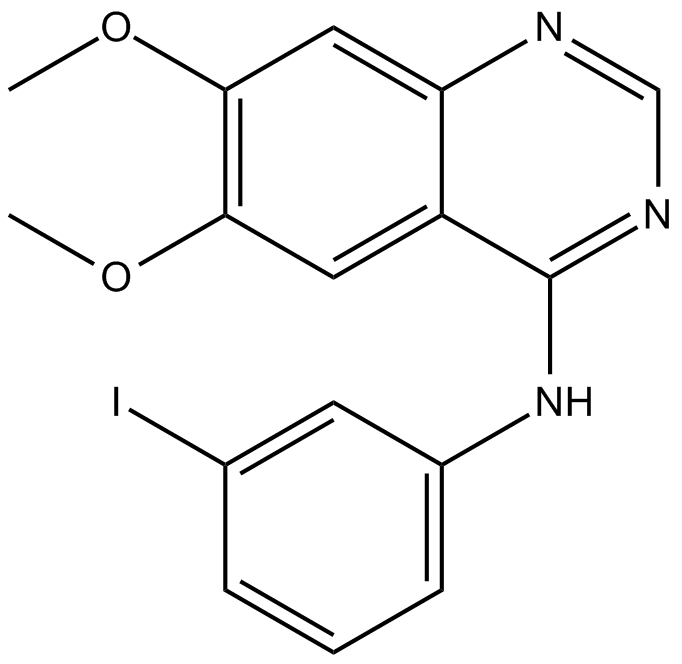 C5417 AG-1557Summary: 表皮生长因子受体(EGFR)酪氨酸激酶抑制剂
C5417 AG-1557Summary: 表皮生长因子受体(EGFR)酪氨酸激酶抑制剂 -
 C4953 HA-100 (hydrochloride)Summary: 蛋白激酶(PKs)抑制剂
C4953 HA-100 (hydrochloride)Summary: 蛋白激酶(PKs)抑制剂

