Serine Protease
Serine proteases, named for the nucleophilic Ser residue at the active site, are a large and diverse group of proteases (accounting for approximately one-third of all proteases) that are characterized by the presence of the Asp-His-Ser “charge relay” system (catalytic triad) in their chemical structures. However, with the development of technology, a variety of novel serine proteases with catalytic triads and dyads have been discovered, including Ser-His-Glu, Ser-Lys/His, His-Ser-His and N-terminal Ser. Serine proteases are traditionally divided into four clans based on the Asp-His-Ser triad in different structural contexts, including chymotrypsin-like proteases, subtilisin-like proteases, carboxypeptidase Y-like proteases and Clp protease-like proteases.
-
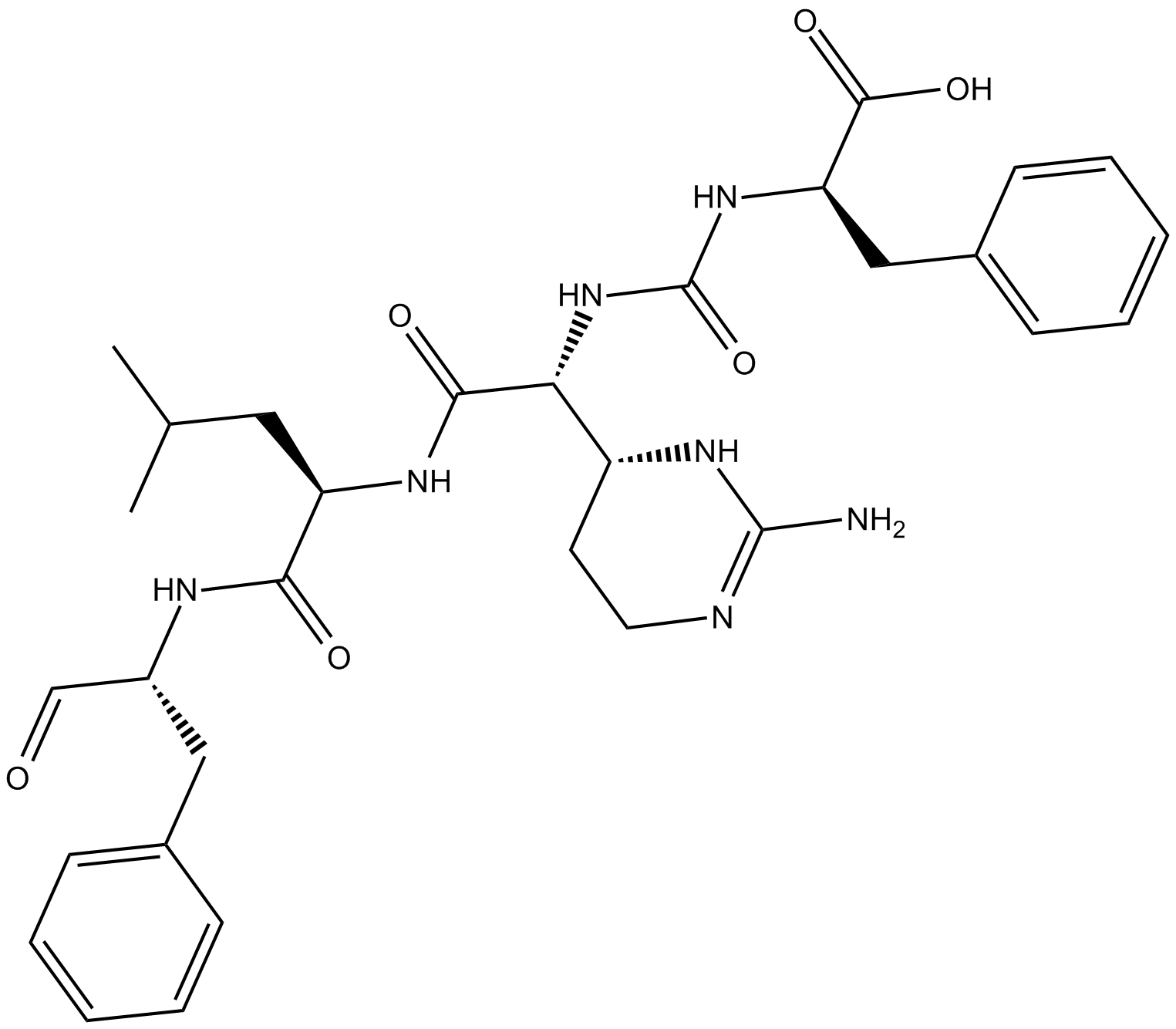 C5589 ChymostatinSummary: 糜蛋白酶样丝氨酸蛋白酶抑制剂
C5589 ChymostatinSummary: 糜蛋白酶样丝氨酸蛋白酶抑制剂 -
 C5839 Tosyllysine Chloromethyl Ketone (hydrochloride)Summary: 丝氨酸蛋白酶抑制剂
C5839 Tosyllysine Chloromethyl Ketone (hydrochloride)Summary: 丝氨酸蛋白酶抑制剂 -
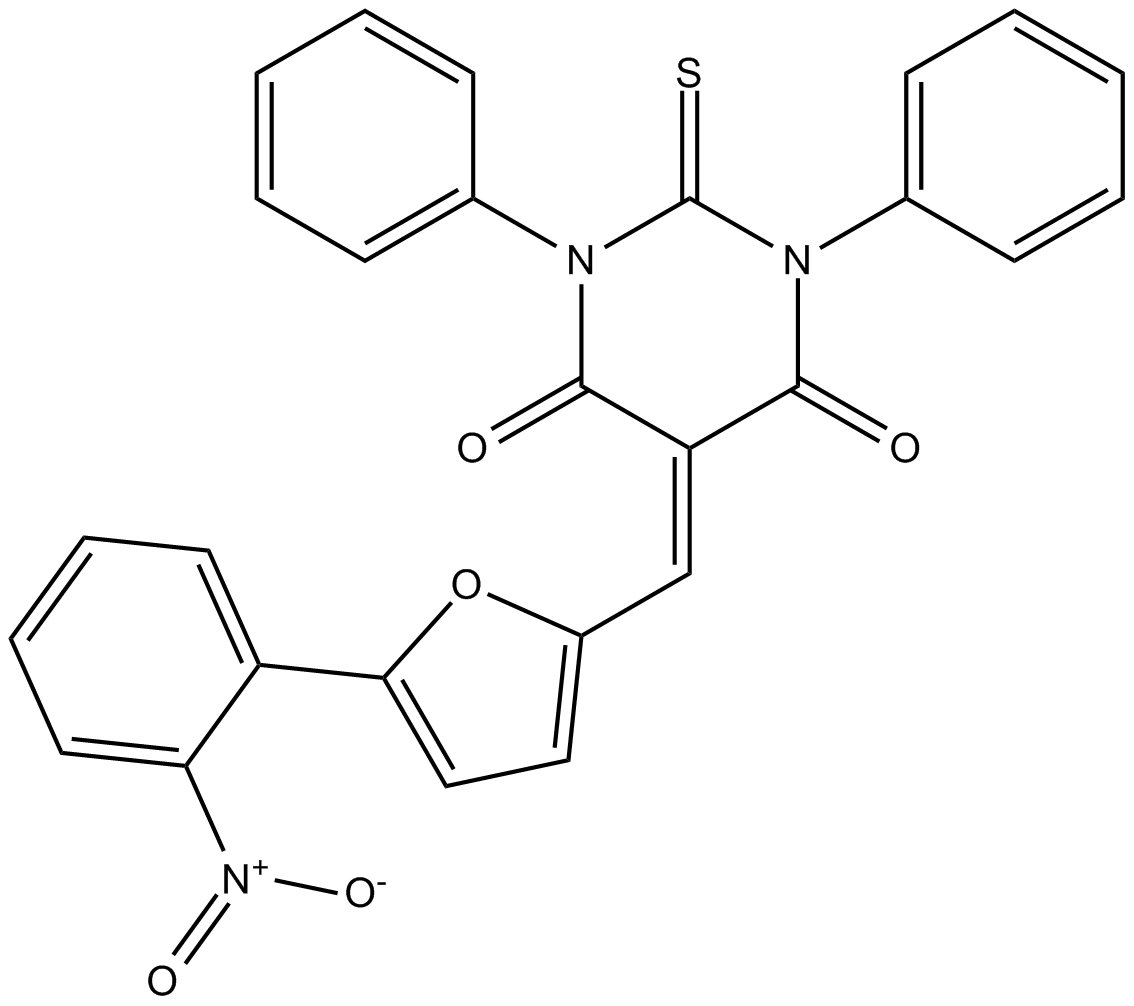 C4437 UCF 101Summary: Omi/HtrA2蛋白水解活性的抑制剂
C4437 UCF 101Summary: Omi/HtrA2蛋白水解活性的抑制剂 -
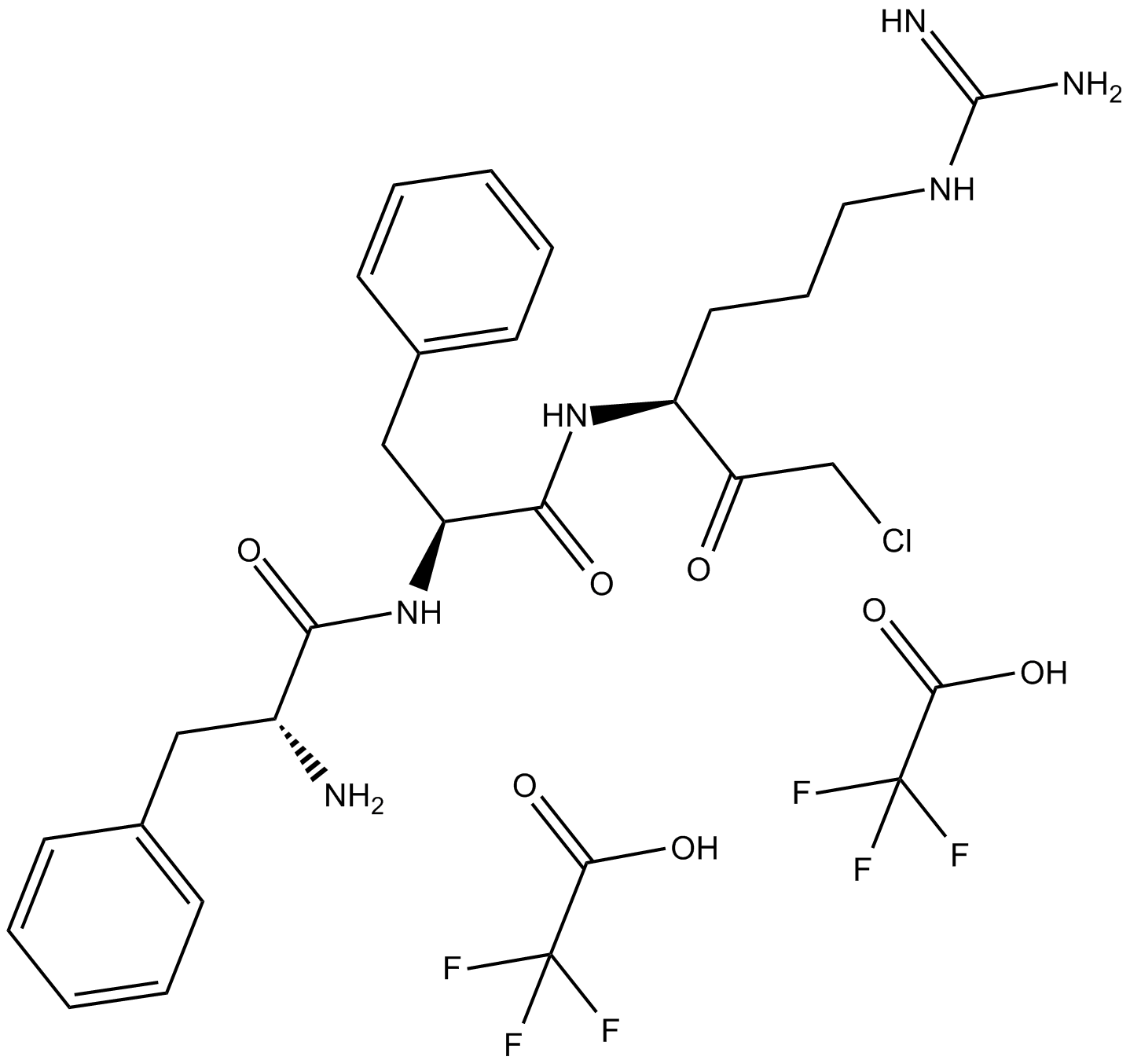 C3393 PPACKII (trifluoroacetate salt)Summary: 腺体和血浆激肽释放酶抑制剂
C3393 PPACKII (trifluoroacetate salt)Summary: 腺体和血浆激肽释放酶抑制剂 -
 A2587 PMSF1 Citation中文名: 苯甲基磺酰氟Target: Serine ProteasesSummary: 丝氨酸蛋白酶不可逆抑制剂
A2587 PMSF1 Citation中文名: 苯甲基磺酰氟Target: Serine ProteasesSummary: 丝氨酸蛋白酶不可逆抑制剂 -
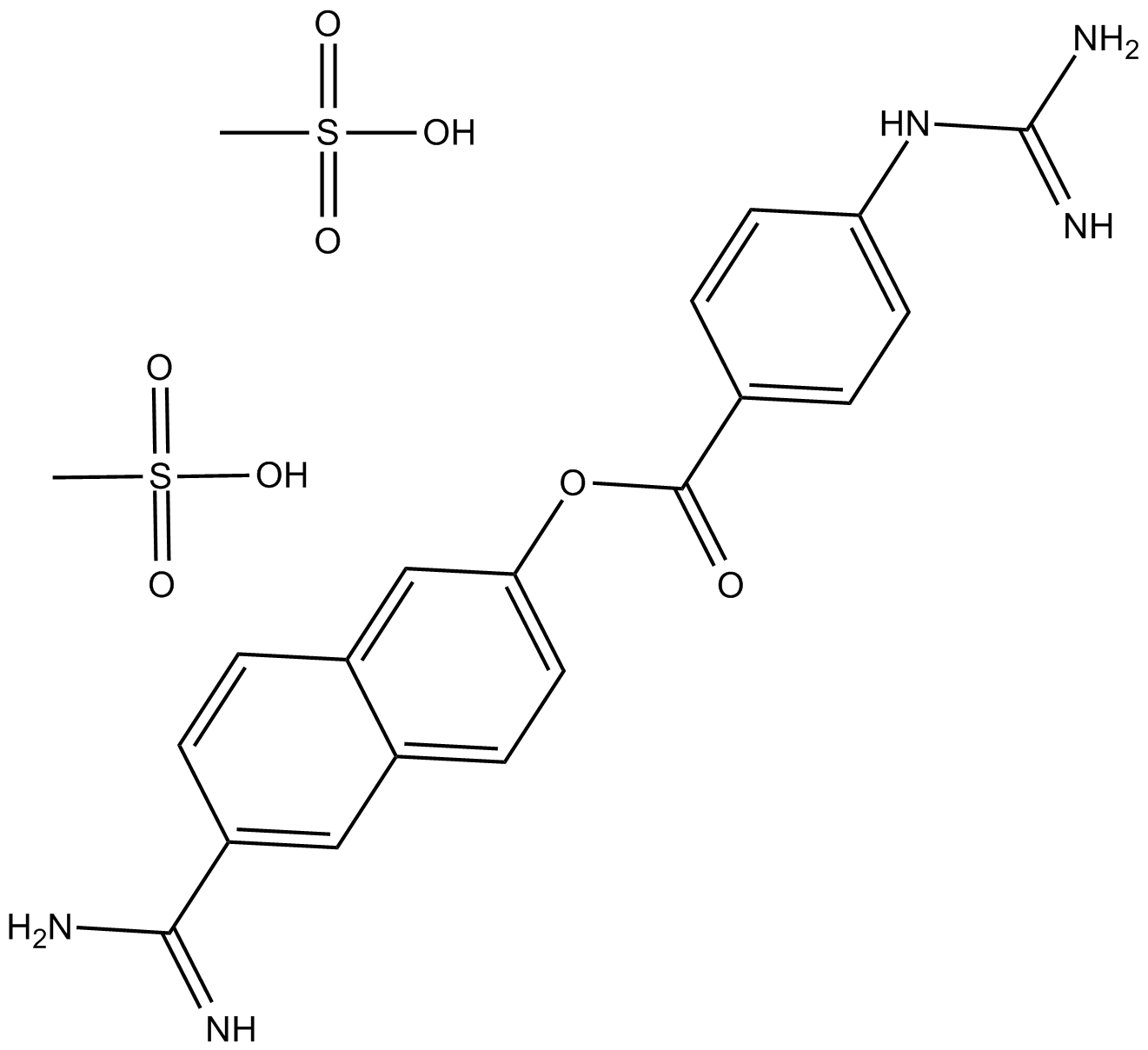 A2586 Nafamostat Mesylate(FUT-175)中文名: 甲磺酸萘莫司他Summary: 丝氨酸蛋白酶抑制剂
A2586 Nafamostat Mesylate(FUT-175)中文名: 甲磺酸萘莫司他Summary: 丝氨酸蛋白酶抑制剂 -
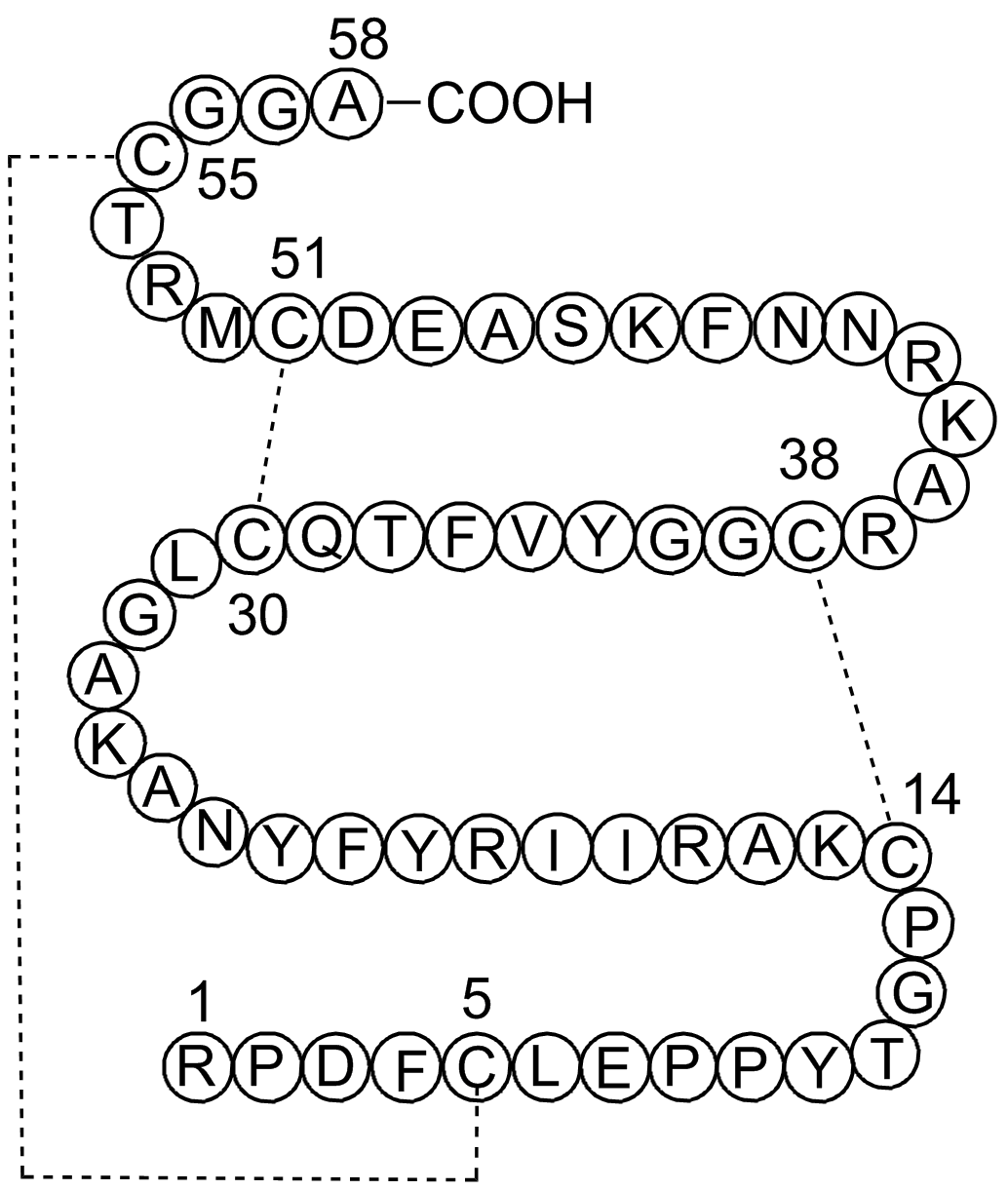 A2574 Aprotinin1 Citation中文名: 抑酶肽Target: Trypsin|Chymotrypsin|Kallikrein|TrypsinogenSummary: 牛胰蛋白酶抑制剂
A2574 Aprotinin1 Citation中文名: 抑酶肽Target: Trypsin|Chymotrypsin|Kallikrein|TrypsinogenSummary: 牛胰蛋白酶抑制剂 -
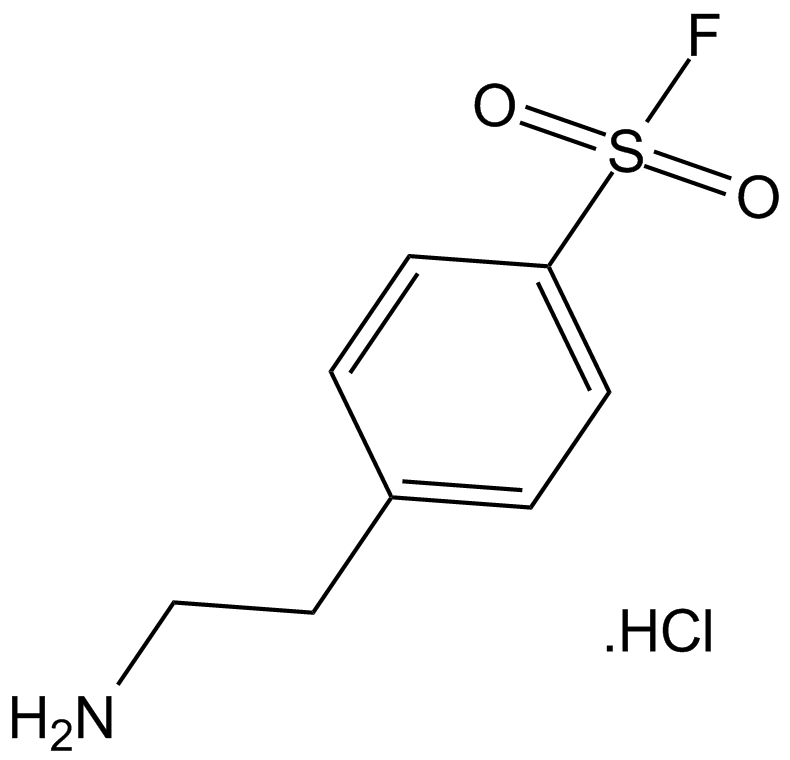 A2573 AEBSF.HCl1 CitationTarget: Serine ProteasesSummary: 丝氨酸蛋白酶抑制剂
A2573 AEBSF.HCl1 CitationTarget: Serine ProteasesSummary: 丝氨酸蛋白酶抑制剂 -
 A2570 Leupeptin, Microbial中文名: 亮抑酶肽Target: Cathepsins|Calpains|TrypsinsSummary: 丝氨酸和半胱氨酸蛋白酶抑制剂
A2570 Leupeptin, Microbial中文名: 亮抑酶肽Target: Cathepsins|Calpains|TrypsinsSummary: 丝氨酸和半胱氨酸蛋白酶抑制剂

