Ubiquitination/ Proteasome

Once the substrate protein is labeled, proteasome will bind to a polyubiquitin chain, allowing the degradation of the labeled protein. The polyubiquitinated target protein is then recognized and degraded by the 26S proteasome. Deubiquitinating enzymes (DUBs) reverse the process of ubiquitination by removing ubiquitin from its substrate protein. Dysregulation of the ubiquitin-proteasome system has been linked to cancer, diabetes, cardiovascular and neurodegenerative diseases etc.
-
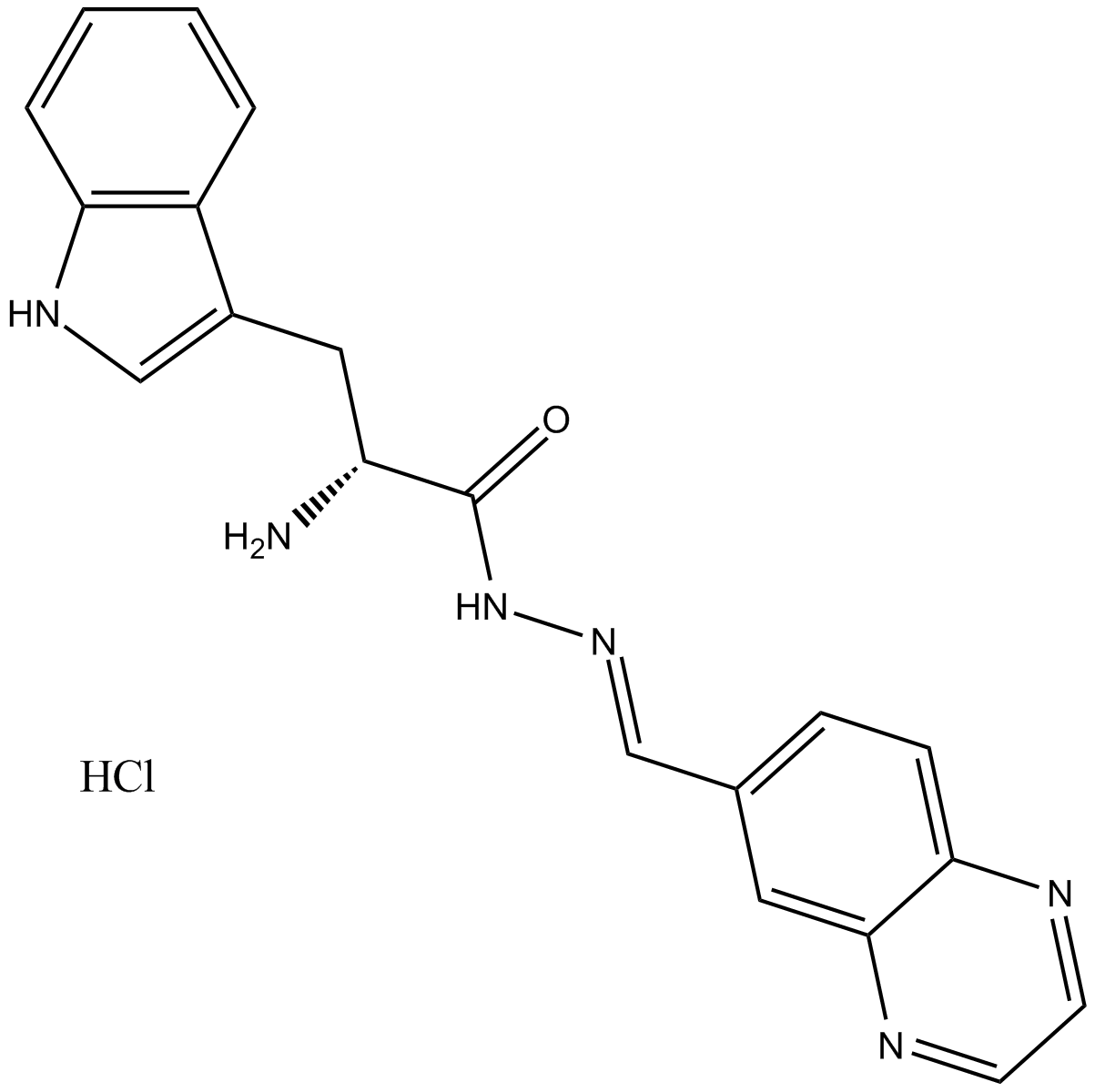 B8557 Rhosin hydrochlorideSummary: 有效和特异性的RhoA subfamily Rho GTPases抑制剂
B8557 Rhosin hydrochlorideSummary: 有效和特异性的RhoA subfamily Rho GTPases抑制剂 -
 B6032 CB-5083Target: p97Summary: p97抑制剂
B6032 CB-5083Target: p97Summary: p97抑制剂 -
 B2151 STF-62247Target: AutophagySummary: 肾细胞自噬诱导剂
B2151 STF-62247Target: AutophagySummary: 肾细胞自噬诱导剂 -
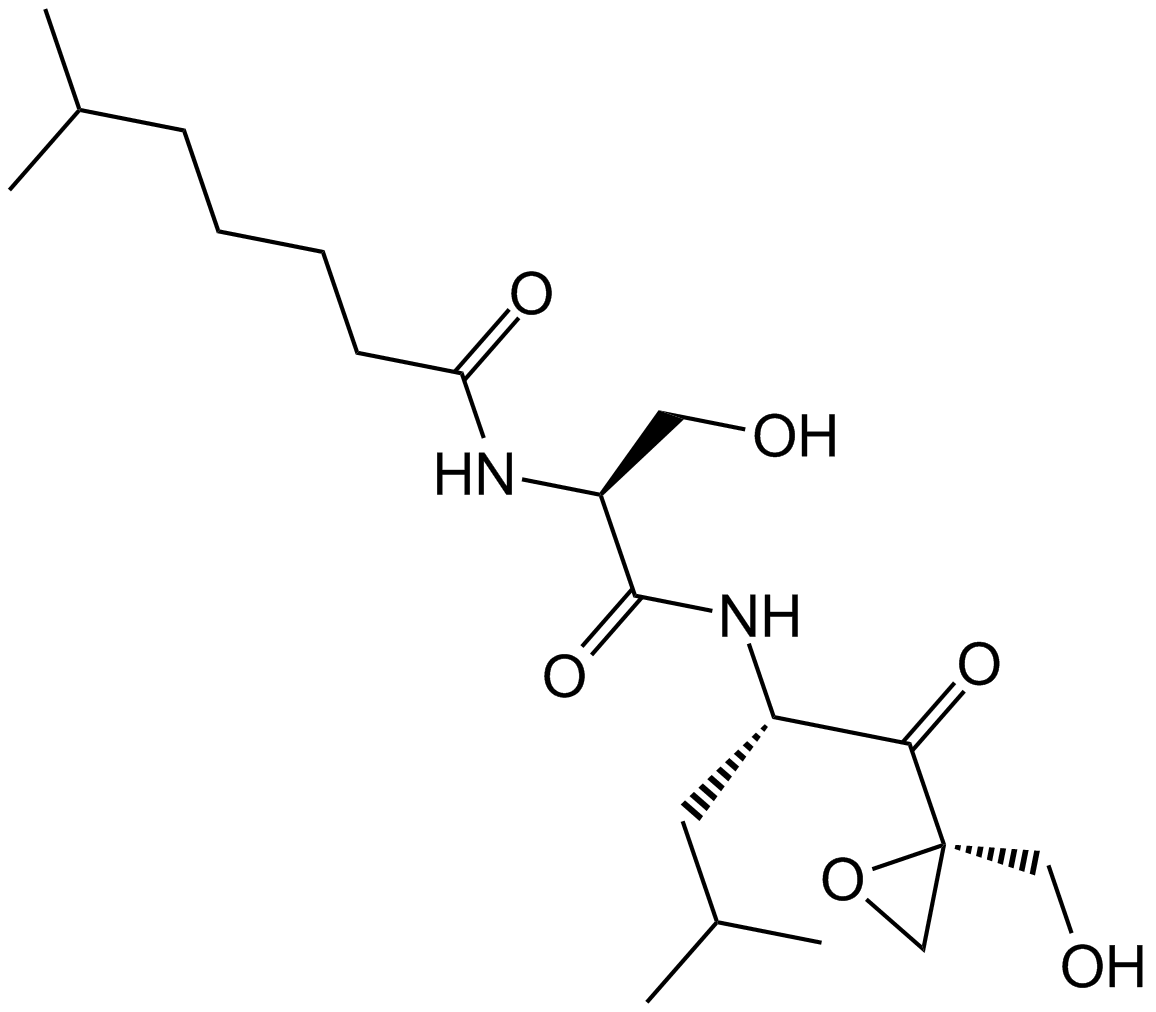 A8172 Dihydroeponemycin1 CitationSummary: 蛋白酶体抑制剂,抗癌剂。
A8172 Dihydroeponemycin1 CitationSummary: 蛋白酶体抑制剂,抗癌剂。 -
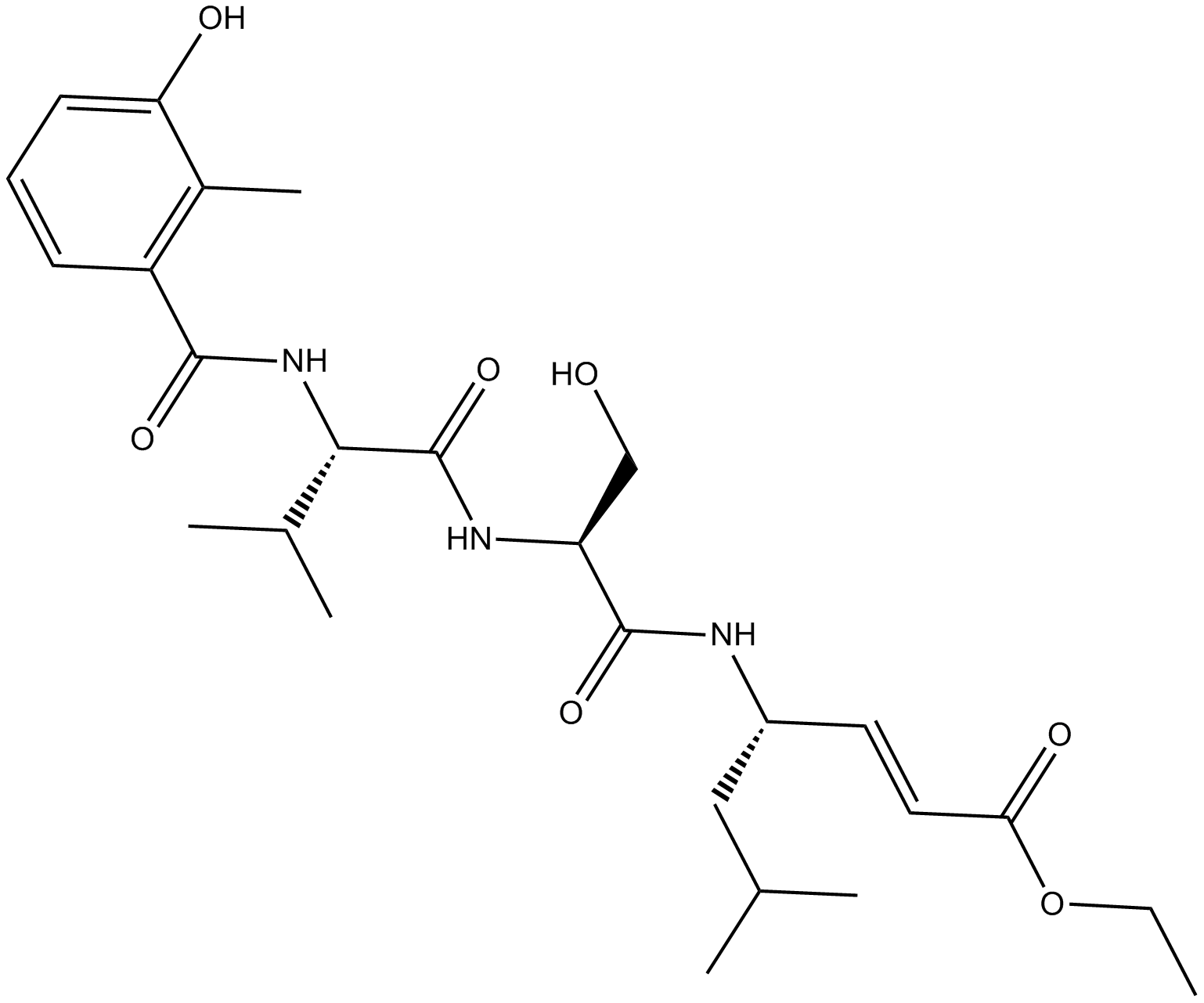 C4090 HMB-Val-Ser-Leu-VESummary: 20S蛋白酶体的胰蛋白酶样活性抑制剂
C4090 HMB-Val-Ser-Leu-VESummary: 20S蛋白酶体的胰蛋白酶样活性抑制剂 -
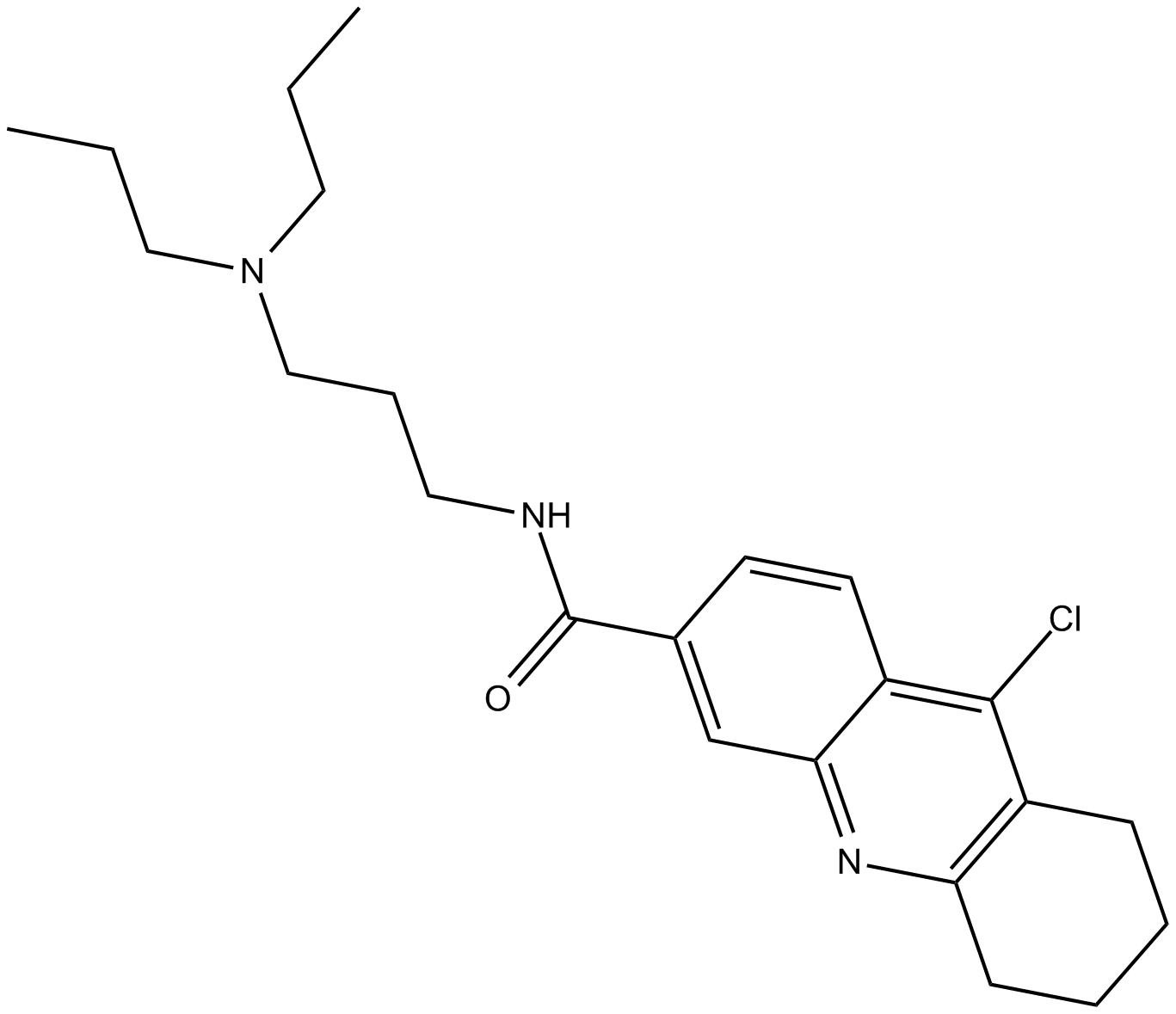 A8740 C598-0466Summary: USP7抑制剂
A8740 C598-0466Summary: USP7抑制剂 -
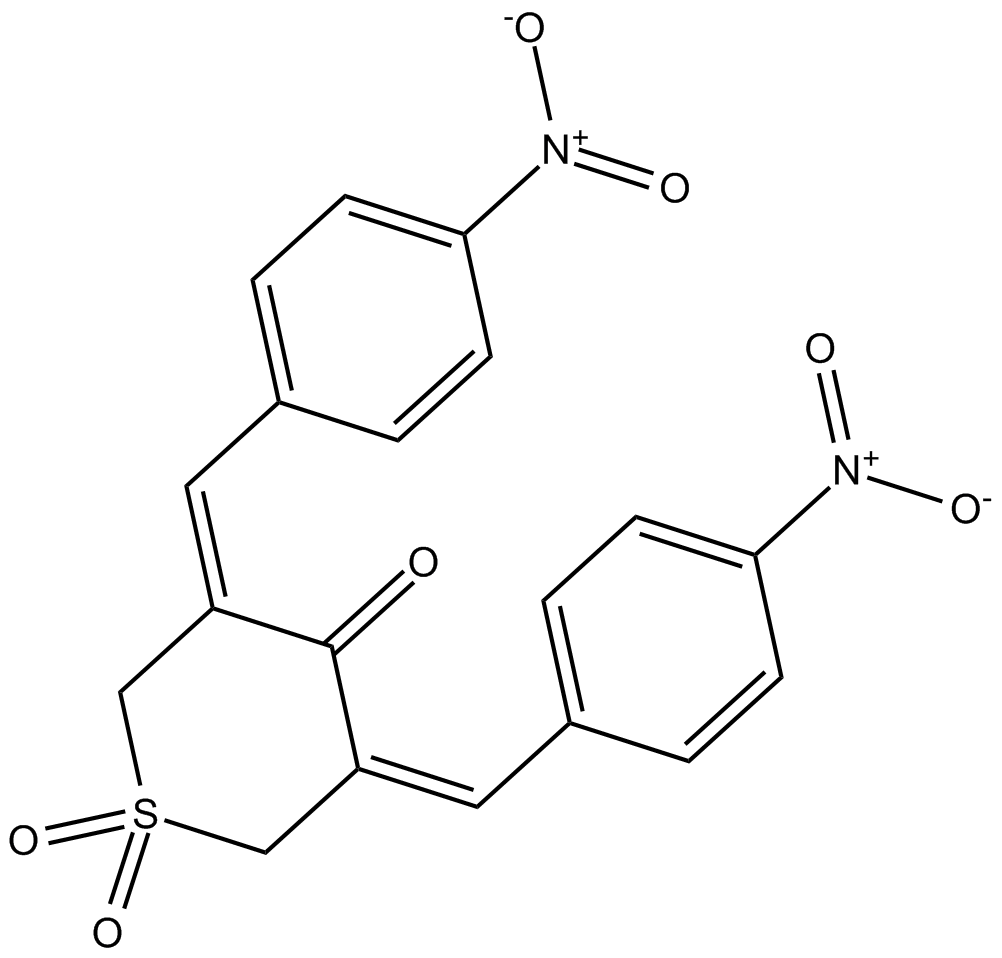 C3250 Ubiquitin Isopeptidase Inhibitor ISummary: 泛素异肽酶抑制剂
C3250 Ubiquitin Isopeptidase Inhibitor ISummary: 泛素异肽酶抑制剂 -
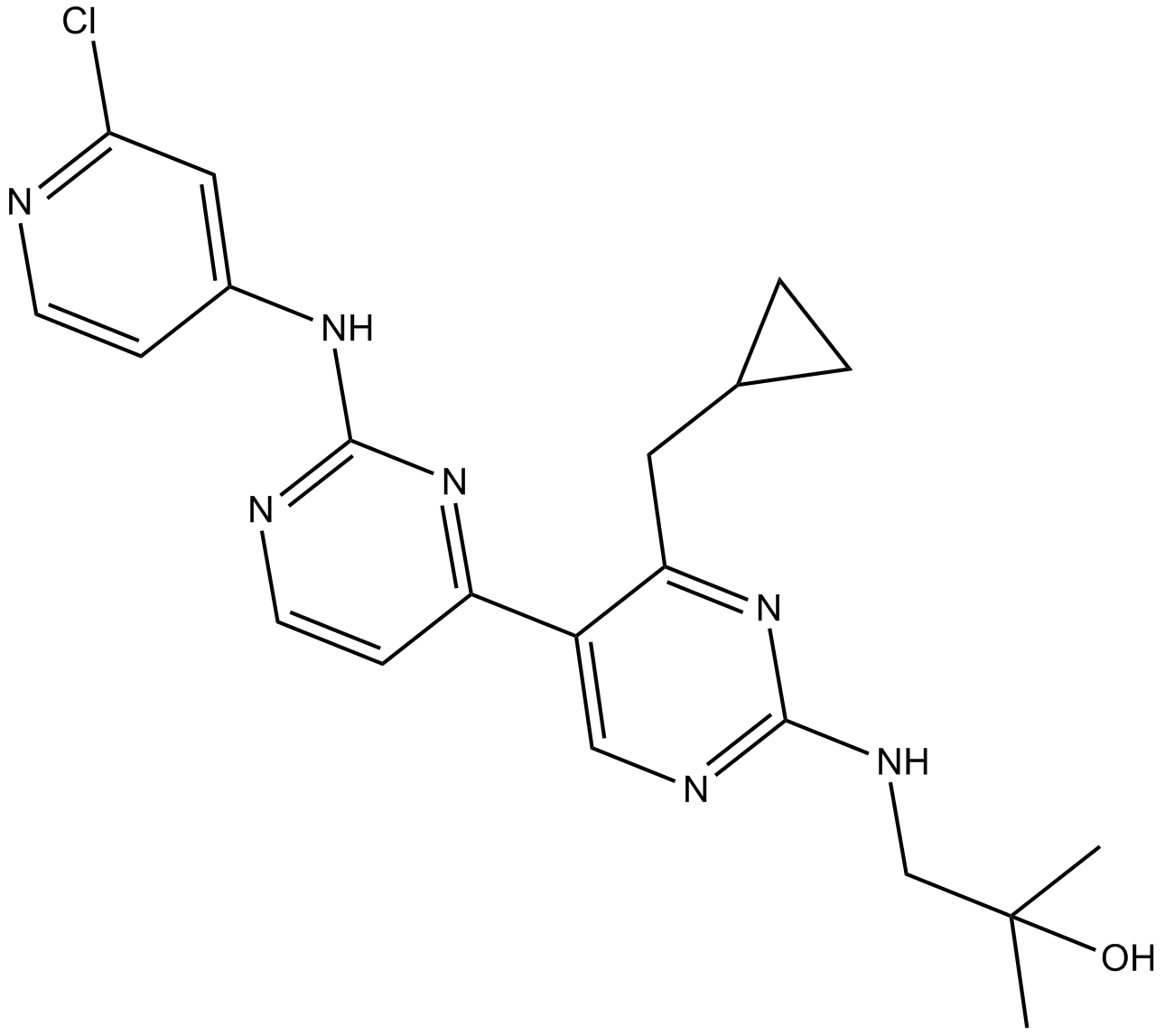 B6179 VPS34-IN1Summary: Vps34抑制剂
B6179 VPS34-IN1Summary: Vps34抑制剂 -
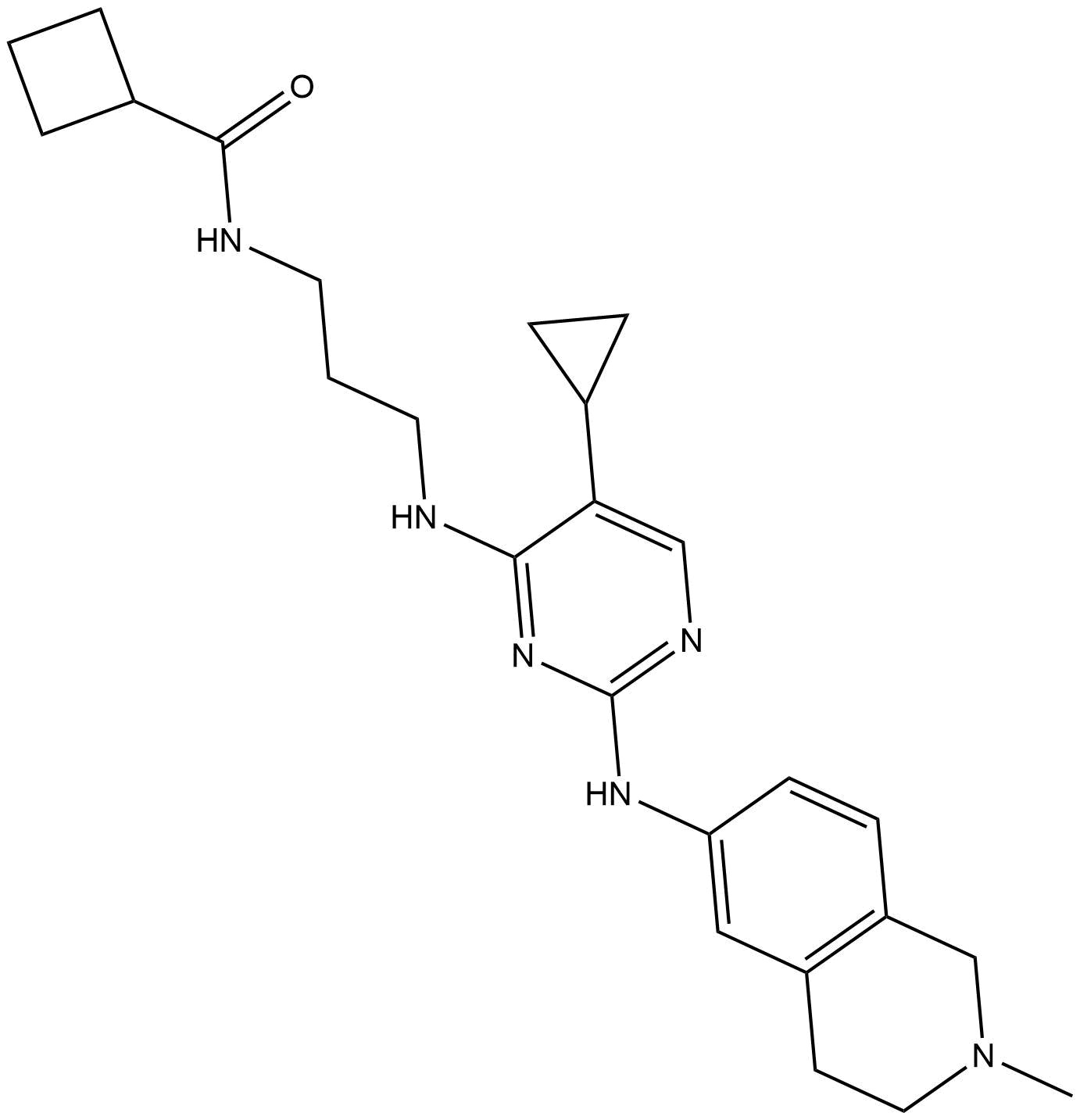 B6174 MRT68921Summary: 双自噬激酶ULK1/2抑制剂
B6174 MRT68921Summary: 双自噬激酶ULK1/2抑制剂 -
 B6160 PIK-III1 CitationSummary: VPS34抑制剂并抑制自噬
B6160 PIK-III1 CitationSummary: VPS34抑制剂并抑制自噬

