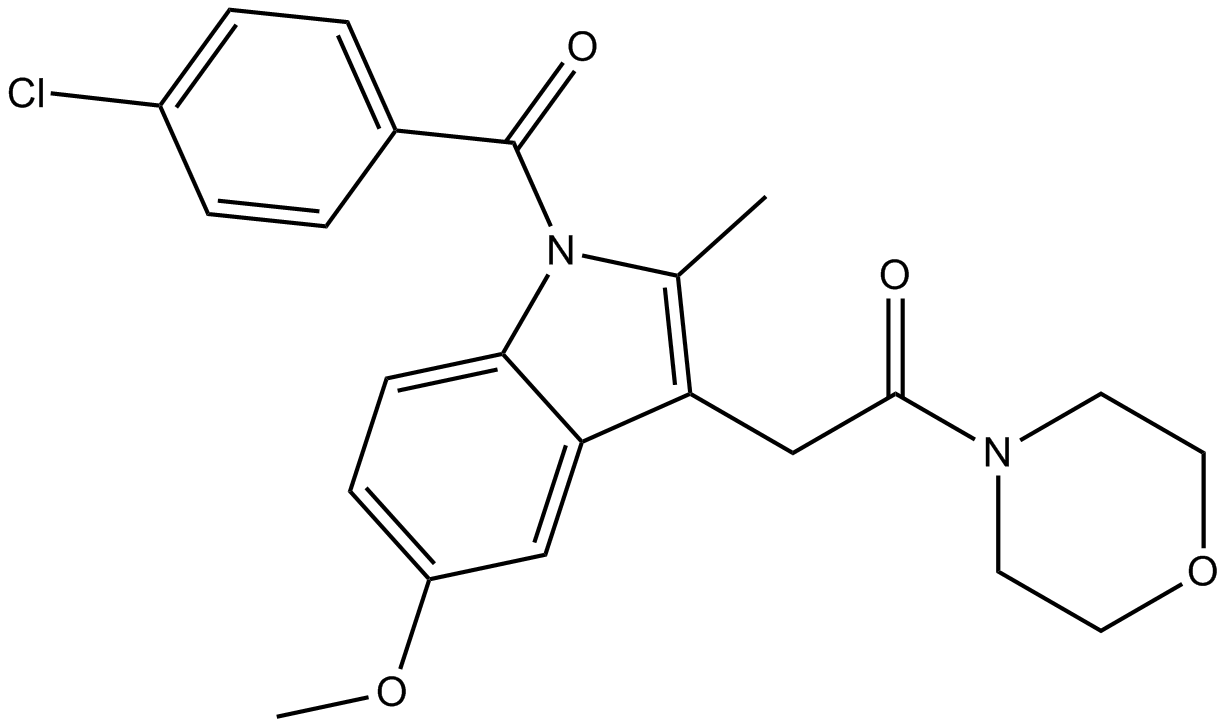
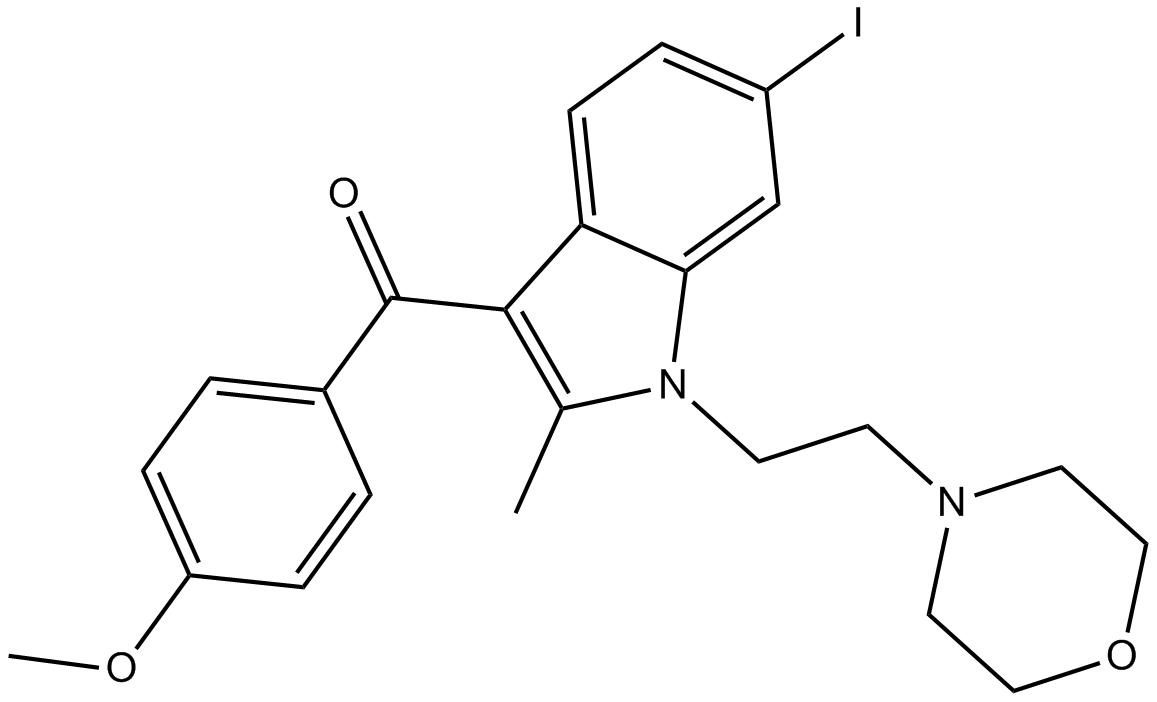
AM1241
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
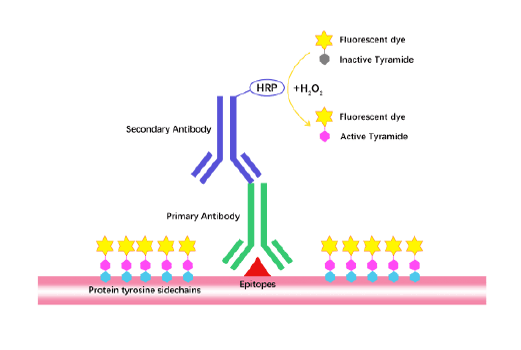
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
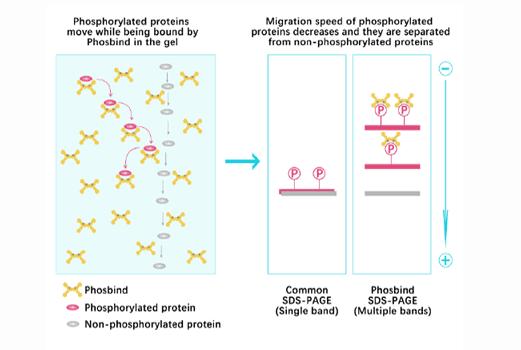
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
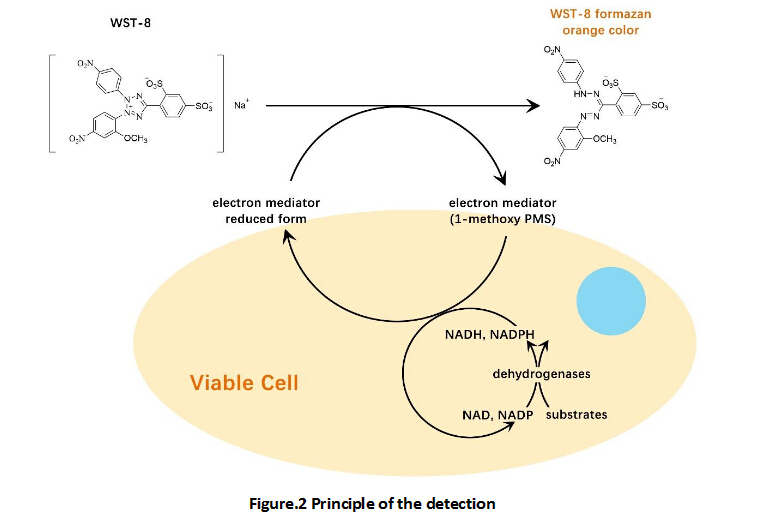
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
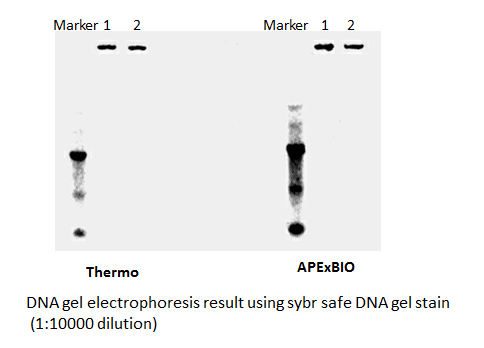
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
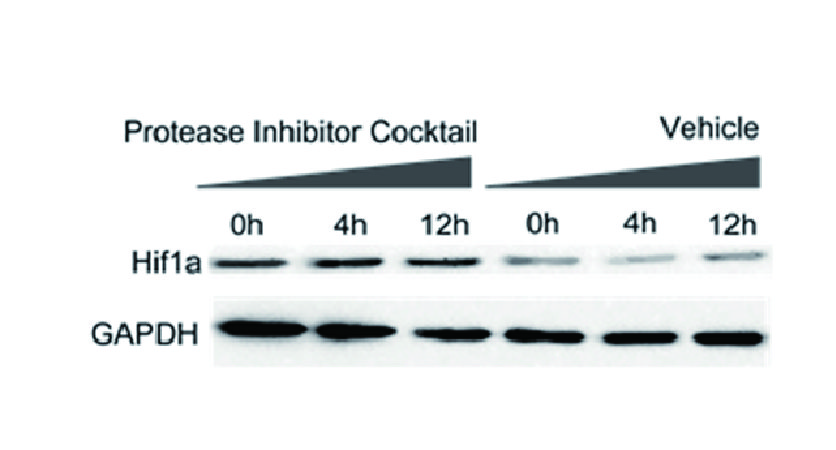
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
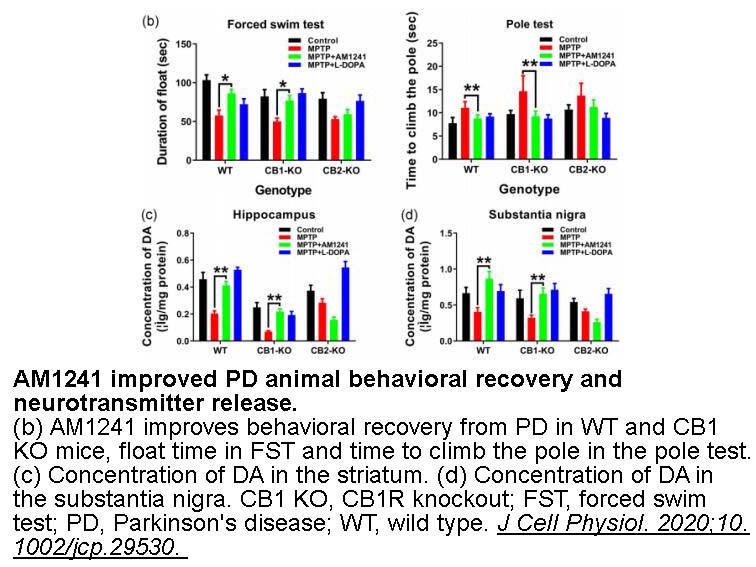
2型大麻素受体,缩写为CB2,是由人体内CNR2基因编码的大麻素受体家族的G蛋白偶联受体。CB2受体对于神经退行性疾病可能有一定的治疗作用,如阿尔茨海默氏病。AM-1241是来自于aminoalkylindole家族的化合物,作为大麻素受体CB2有效和选择性的激动剂,在动物研究中发挥镇痛效果,特别是非典型疼痛如痛觉过敏和异常性疼痛。
体外实验:研究的目的是提供R,S-AM1241及其拆分对映体在体外和体内的表征。在cAMP的抑制实验中,R,S-AM1241被发现是人CB2受体的激动剂,是大鼠和小鼠CB2受体的反向激动剂。R-AM1241对三个CB2受体的亲和力比S-AM1241高40倍,并表现出类似于外消旋体的功能性分布。相比之下,S-AM1241是三种CB2受体的激动剂。在疼痛模型中,S-AM1241比R-AM1241或任一外消旋物更有效。拮抗剂封锁表明,S-AM1241的体内效果是由CB2受体介导的。这些发现构成了R,S- AM1241对啮齿类CB2受体功能的第一次体外评估,以及在重组细胞系统和体内对AM1241对映体进行第一次特征性描述。S-AM1241,AM1241的功能性CB2激动剂对映体,具有镇痛功效,这与之前研究发现的CB2激动剂能有效减轻疼痛相一致[2]。
在体实验:对于肌萎缩侧索硬化症(ALS)的治疗仍然遥遥无期。运动神经元退化是ALS的主要病理学特征,然而非神经元细胞也能促进疾病的进程。比如,炎症性反应在这个过程中发挥了重要的作用。AM1241是大麻素CB2受体的选择性激动剂,对于炎症和痛觉过敏模型是有效的。在ALS小鼠(hSOD1G93A转基因小鼠)发病时,AM1241治疗具有延缓疾病进展的迹象。与对照组相比,药物推迟了发病时给药组小鼠的运动功能障碍。使用数学模型分析ALS的三个条件,即运动功能的丧失、麻痹评分和体重损失。雄性小鼠中,AM1241给药12.5天后,推迟了运动功能(在一个旋转杆进行评估)的损失。在雌性小鼠中,AM1241给药后,尽管没有显著的统计学意义,仍然延长了3天通过旋转杆的能力。在雄性小鼠中,AM1241也延长了5天的时间,达到50%的可视觉评估的点。AM1241没有影响体重的损失和存活(129.8 ± 1.7天,对照;129.1 ± 7.0天, AM1241,n=16)。在动物体内,AM1241具有良好的耐受性,大麻素CB2受体选择性化合物可以作为新药开发的基础,用于治疗ALS和其它慢性神经退行性疾病[3]。
临床试验:AM1241仍处于临床前开发阶段,并没有临床研究结果。
参考文献:
[1] Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP Jr. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci U S A. 2003;100(18):10529-33.
[2] B Bingham, PG Jones, AJ Uveges, S Kotnis, P Lu, VA Smith, S-C Sun, L Resnick, M Chlenov, Y He. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. British Journal of Pharmacology (2007) 151, 1061–1070
[3] Kathline Kim, Dan H. Moore, Alexandros Makriyannis, Mary E. Abood. AM1241, a cannabinoid CB2 receptor selective compound, delays disease progression in a mouse model of amyotrophic lateral sclerosis. European Journal of Pharmacology 542 (2006) 100-105
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 503.33 |
| Cas No. | 444912-48-5 |
| Formula | C22H22IN3O3 |
| Solubility | ≥50.3 mg/mL in DMSO with gentle warming; insoluble in H2O; ≥3.87 mg/mL in EtOH with ultrasonic |
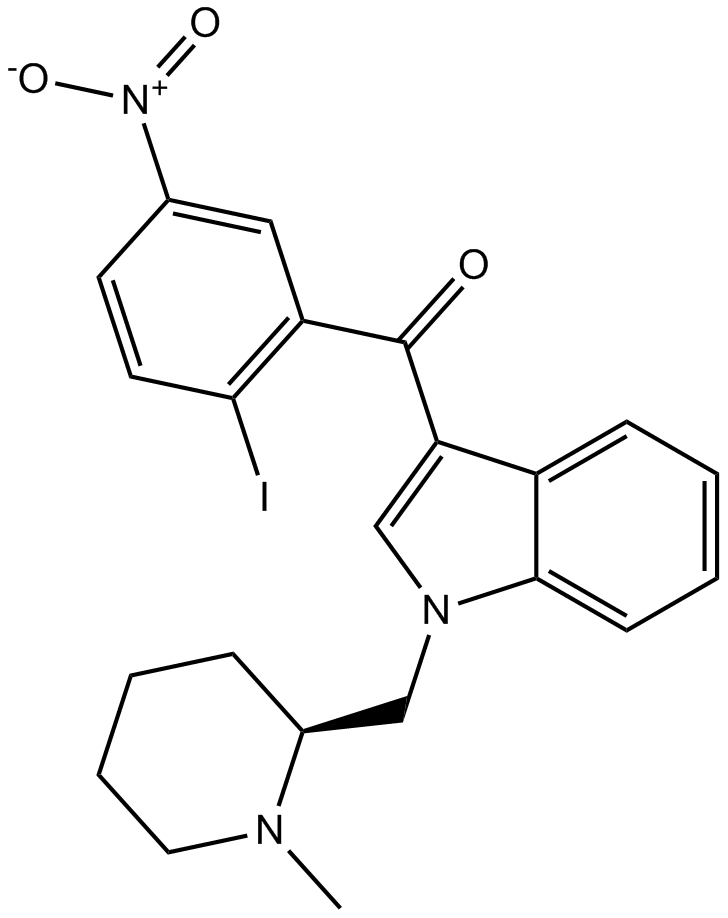
| Chemical Name | (2-iodo-5-nitrophenyl)-[1-[(1-methylpiperidin-2-yl)methyl]indol-3-yl]methanone |
| SDF | Download SDF |
| Canonical SMILES | CN1CCCCC1CN2C=C(C3=CC=CC=C32)C(=O)C4=C(C=CC(=C4)[N+](=O)[O-])I |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 激酶实验 [1]: | |
|
结合实验 |
从稳定表达人CB2受体的HEK细胞或稳定表达人CB1受体的CHO细胞系制备膜样品。在蛋白酶抑制剂存在下,在含有50 mM Tris-HCl、pH 7.4、1 mM MgCl2和1 mM EDTA的缓冲液中,使用Polytron进行2×10秒脉冲,收获细胞并匀化,然后以45000g离心20分钟将膜沉淀物以-80℃等份洗涤并冷冻。在含有50 mM Tris-HCl、pH 7.4、2.5 mM EDTA、5 mM MgCl2和0.05%无脂肪酸牛血清白蛋白(BSA)的测定缓冲液中,使用[3H]CP 55,940(0.01-8 nm)在30℃下进行饱和结合反应90分钟,通过UniFilter-96 GF/C过滤板快速真空过滤终止反应,并用冷测定缓冲液洗涤四次。在测试化合物(0.1 nM-10μM)存在下,使用0.5 nM [3H] CP 55,940进行竞争实验。非特异性结合由10μM未标记的CP 55,940定义。 |
| 细胞实验 [1]: | |
|
细胞系 |
稳定表达人CB2受体的人胚肾(HEK)细胞,稳定表达人CB1受体的中国仓鼠卵巢(CHO)细胞系 |
|
溶解方法 |
该化合物在DMSO中的溶解度大于25.2 mg/mL。若获取更高浓度的溶液,可在37℃下孵育10分钟,随后在超声波浴中摇匀。-20℃以下可储存数月。 |
|
反应条件 |
Ki:~7 nM (人CB2受体) |
|
应用 |
在稳定表达人CB2受体的HEK细胞中,AM1241表现出拮抗活性,以浓度依赖性方式阻断激动剂CP 55,940-诱发的Ca2+应答,Kb值为63 nM。在分别表达重组人CB2和CB1受体的稳定的HEK和CHO细胞系膜上,在[3H] CP 55,940竞争结合测定中,AM-1241在人CB2受体上表现出高亲和力,Ki值为约7 nM,而其在人CB1受体上的亲和力较弱,超过80倍。 |
| 动物实验 [2]: | |
|
动物模型 |
成年雄性Sprague-Dawley大鼠 |
|
给药剂量 |
腹腔注射,100或330 μg/kg |
|
应用 |
AM1241(100、330 μg/kg,i.p.)抑制角叉菜胶诱发的热和机械痛觉过敏以及异常性疼痛的发展。在给予角叉菜胶注射的爪中足底注射AM1241(33 μg/kg)抑制了痛觉过敏和异常性疼痛,但在对侧(非发炎)爪施用后却无活性。 |
|
注意事项 |
由于实验环境的不同,实际溶解度可能与理论值略有不同,请测试室内所有化合物的溶解度。 |
|
References: [1]. Yao B B, Mukherjee S, Fan Y, et al. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor[J]. British journal of pharmacology, 2006, 149(2): 145-154. [2]. Nackley A G, Makriyannis A, Hohmann A G. Selective activation of cannabinoid CB 2 receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation[J]. Neuroscience, 2003, 119(3): 747-757. |
|
| 描述 | AM-1241是大麻素CB2受体的选择性激动剂,Ki值为3.4 nM。 | |||||
| 靶点 | CB2 | CB1 | ||||
| IC50 | 3.4 nM(Ki) | 280 nM(Ki) | ||||
化学结构
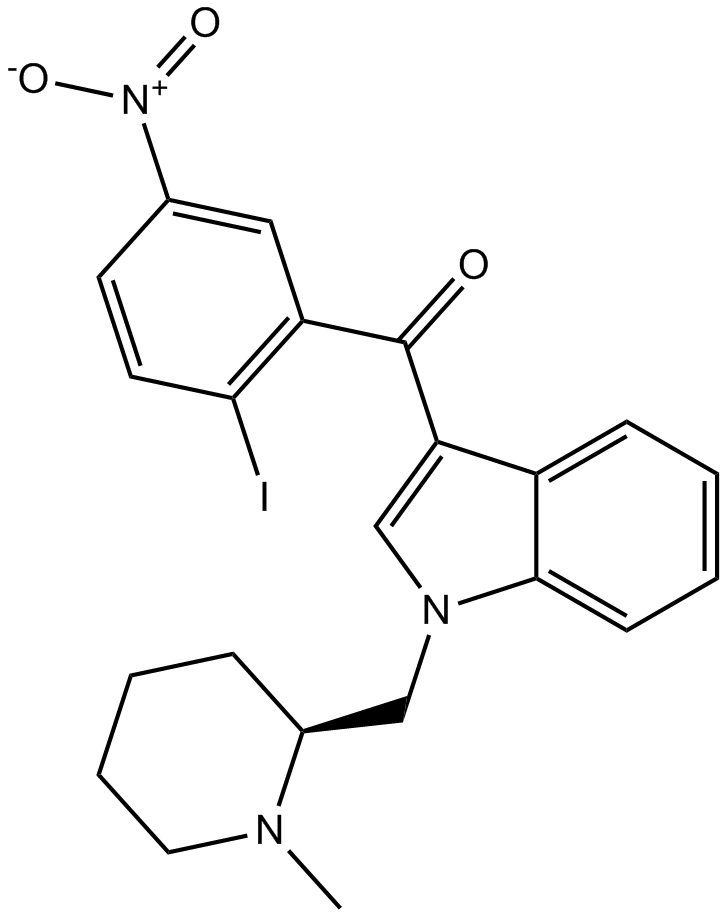
相关生物数据