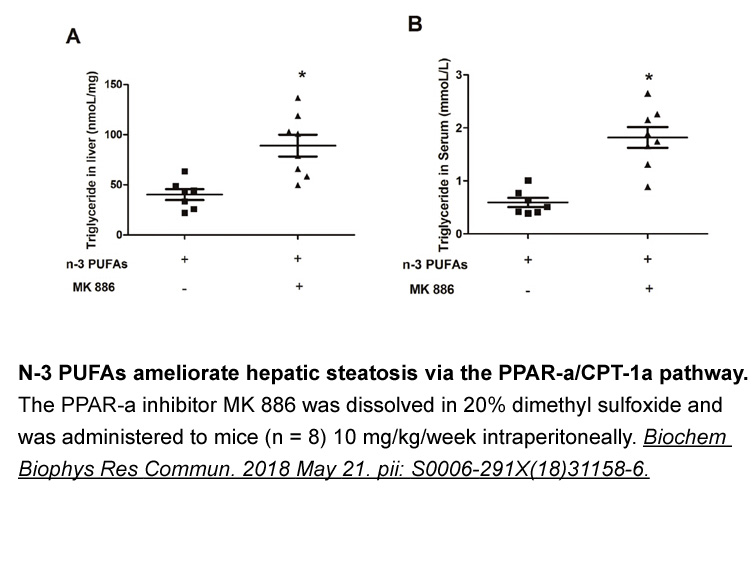
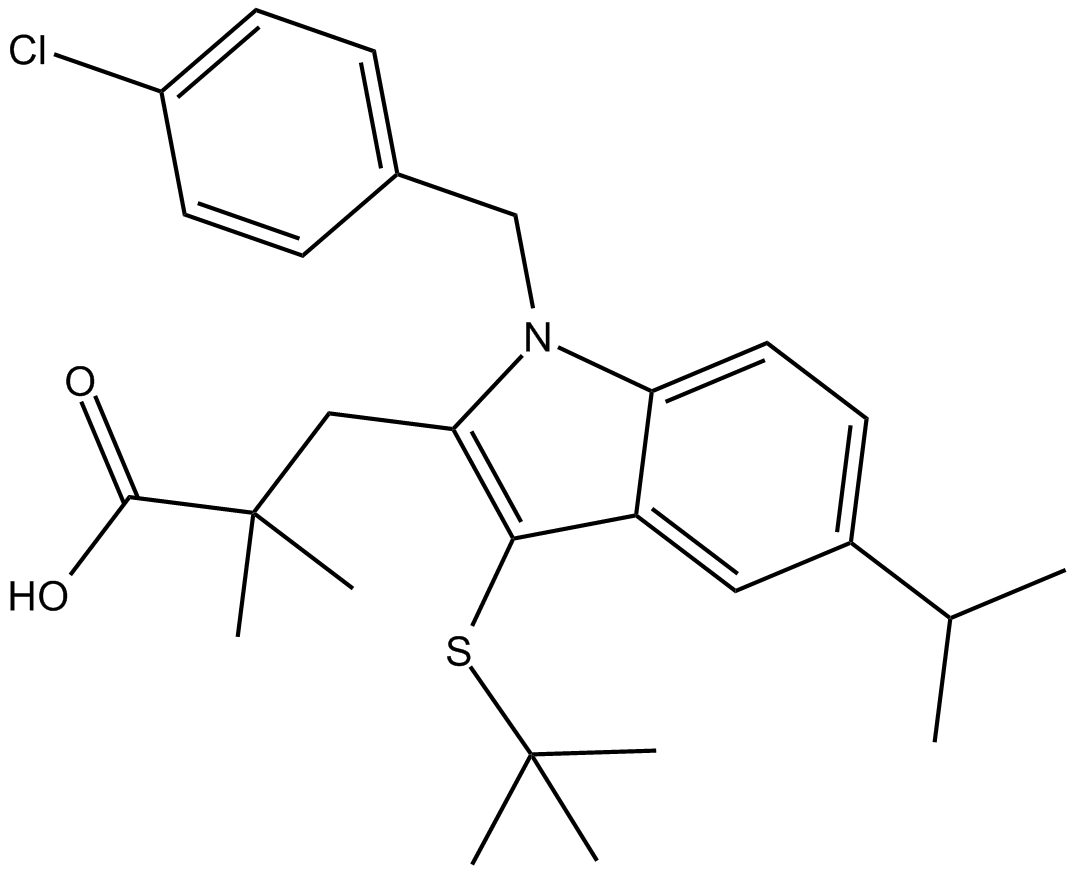
MK 886
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
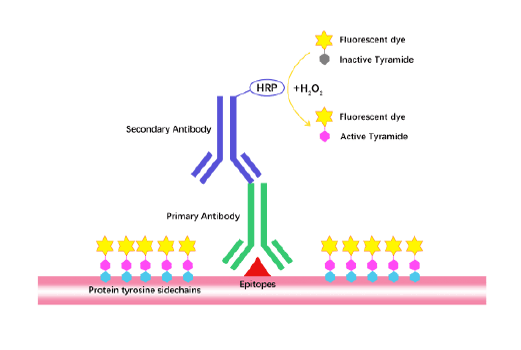
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
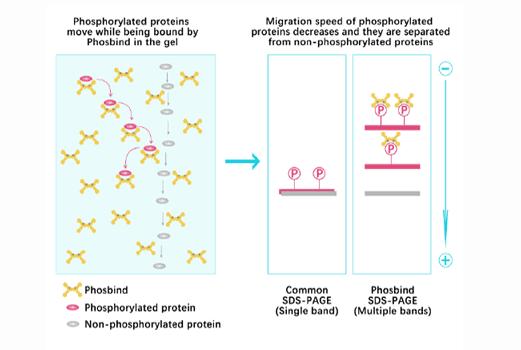
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
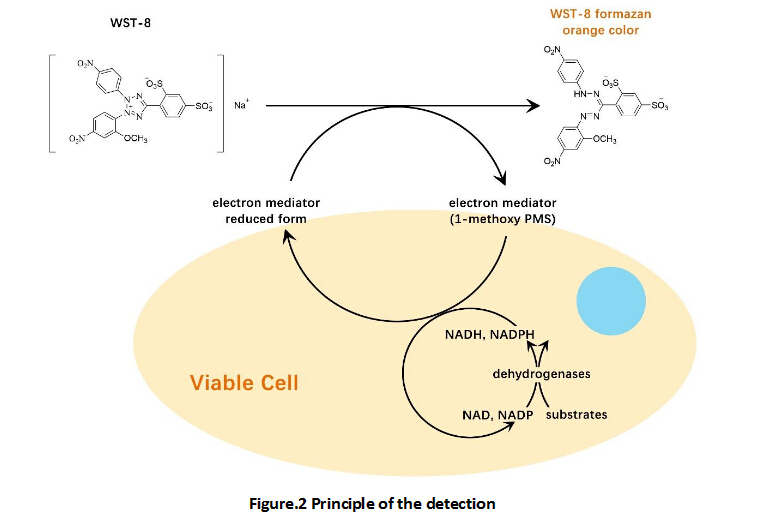
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
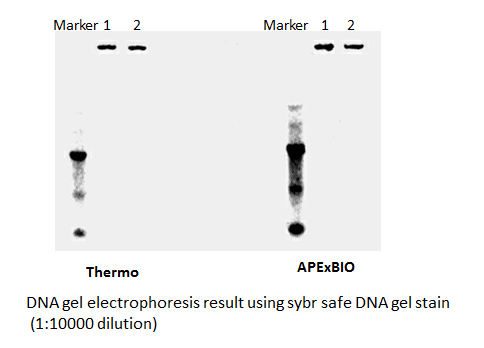
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
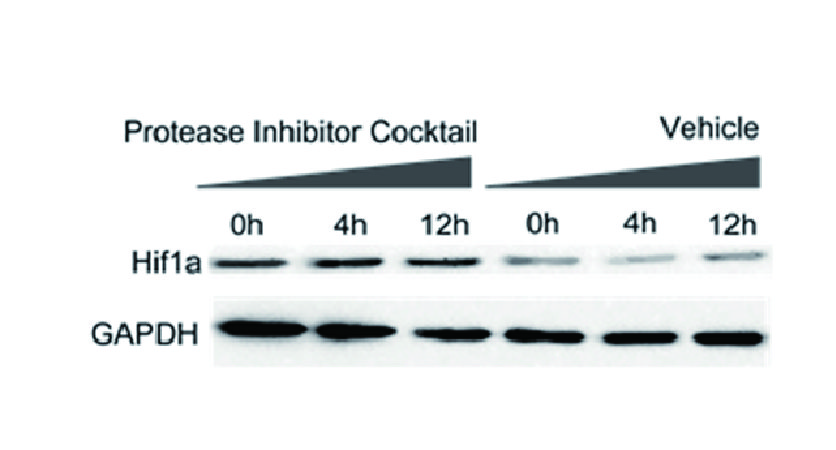
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
MK 886 is a potent, cell-permeable and orally-active inhibitor of 5-lipoxygenase-activating protein (FLAP), with an IC50 value of 30 nM for inhibition of [125I]-L-691,678 photoaffinity labelling. FLAP is essential for the activation of 5-lipoxygenase (5-LO) and therefore for the biosynthesis of leukotrienes. Leukotrienes, the biologically active metabolites of arachidonic acid, have been implicated in various inflammatory responses, such as asthma, arthritis as well as psoriasis. In addition, MK 886 is also a non-competitive antagonist of the peroxisome-proliferator-activated receptor alpha (PPARα), with the ability to induce apoptosis.
References:
1. Mancini JA, Prasit P, Coppolino MG, et al. 5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors. Molecular Pharmacology, 1992, 41(2): 267-272.
2.Dixon RA, Diehl RE, Opas E, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature, 1990, 343(6255): 282-284.
3. Kehrer JP, Biswal SS, La E, et al. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochemical Journal, 2001, 356(Pt 3): 899-906.
4. Imbesi M, Zavoreo I, Uz T, et al. 5-Lipoxygenase inhibitor MK-886 increases GluR1 phosphorylation in neuronal cultures in vitro and in the mouse cortex in vivo. Brain Research, 2007, 1147: 148-153.
| Physical Appearance | White solid |
| Storage | Store at RT |
| M.Wt | 472.08 |
| Cas No. | 118414-82-7 |
| Formula | C27H34ClNO2S |
| Solubility | ≥15.1 mg/mL in DMSO with ultrasonic; ≥2.16 mg/mL in EtOH with ultrasonic; insoluble in H2O |
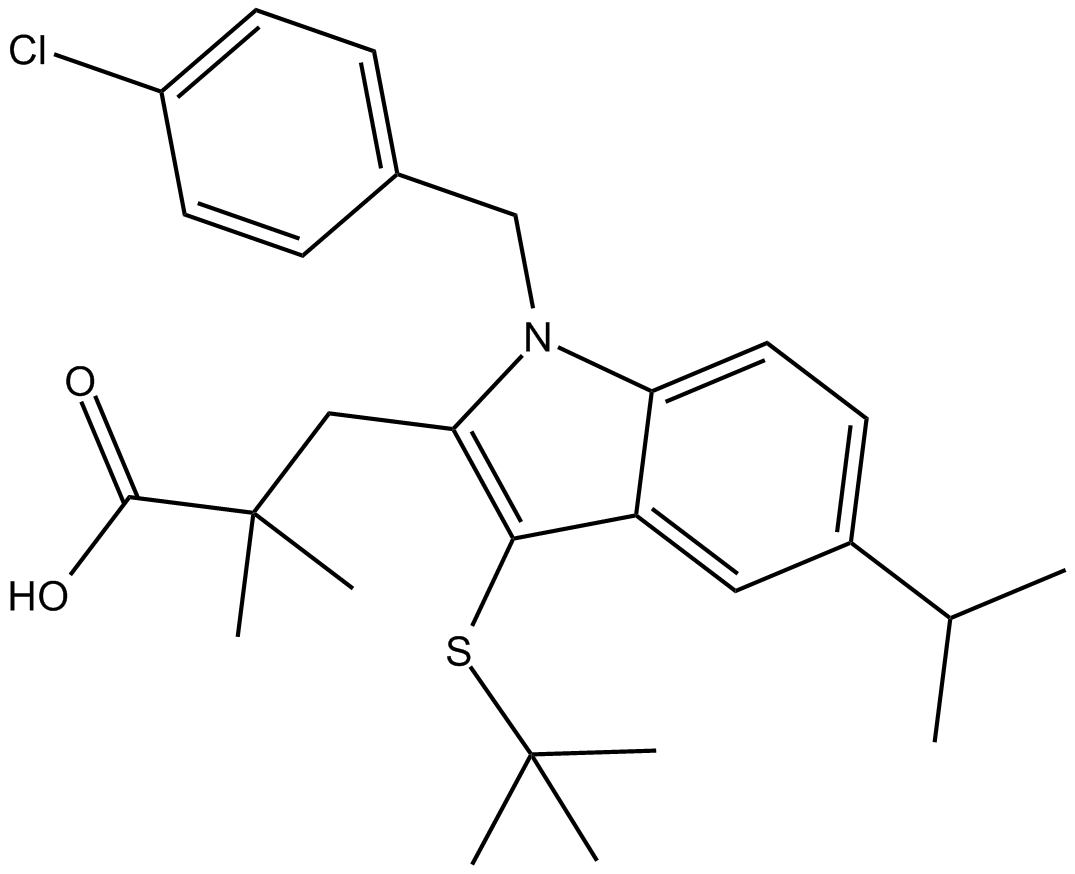
| Chemical Name | 3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-isopropyl-1H-indol-2-yl)-2,2-dimethylpropanoic acid |
| SDF | Download SDF |
| Canonical SMILES | ClC1=CC=C(C=C1)CN(C2=CC=C(C(C)C)C=C32)C(CC(C)(C(O)=O)C)=C3SC(C)(C)C |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| Cell experiment:[2] | |
|
Cell lines |
Osteosarcoma cells expressing 5-LO, 5-LO and rat FLAP (5-LO/FLAP), or rat neutrophils |
|
Reaction Conditions |
0.3 μM MK 886 for osteosarcoma cells and 0.2 μM MK 886 for rat neutrophils |
|
Applications |
MK 886 blocked leukotriene synthesis both in the 5-LO/FLAP cell line and in neutrophils. MK 886 was able to inhibit the synthesis of leukotrienes in intact activated leukocytes, but showed little or no effect on enzymes directly involved in leukotriene synthesis, including 5-LO. |
| Animal experiment:[4] | |
|
Animal models |
Male C57BL/6J mice |
|
Dosage form |
3 mg/kg Intraperitoneal (i.p.) injection |
|
Applications |
Repeated daily i.p. injections of MK 886 increased phosphorylation of AMPAR subunit glutamate receptor 1 (GluR1) in brain samples obtained from the prefrontal cortex, whereas a single injection of MK 886 did not alter cortical GluR1 phosphorylation. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Mancini JA, Prasit P, Coppolino MG, et al. 5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors. Molecular Pharmacology, 1992, 41(2): 267-272. 2.Dixon RA, Diehl RE, Opas E, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature, 1990, 343(6255): 282-284. 3. Kehrer JP, Biswal SS, La E, et al. Inhibition of peroxisome-proliferator-activated receptor (PPAR)alpha by MK886. Biochemical Journal, 2001, 356(Pt 3): 899-906. 4. Imbesi M, Zavoreo I, Uz T, et al. 5-Lipoxygenase inhibitor MK-886 increases GluR1 phosphorylation in neuronal cultures in vitro and in the mouse cortex in vivo. Brain Research, 2007, 1147: 148-153. |
|
质量控制和MSDS
- 批次:
化学结构
相关生物数据