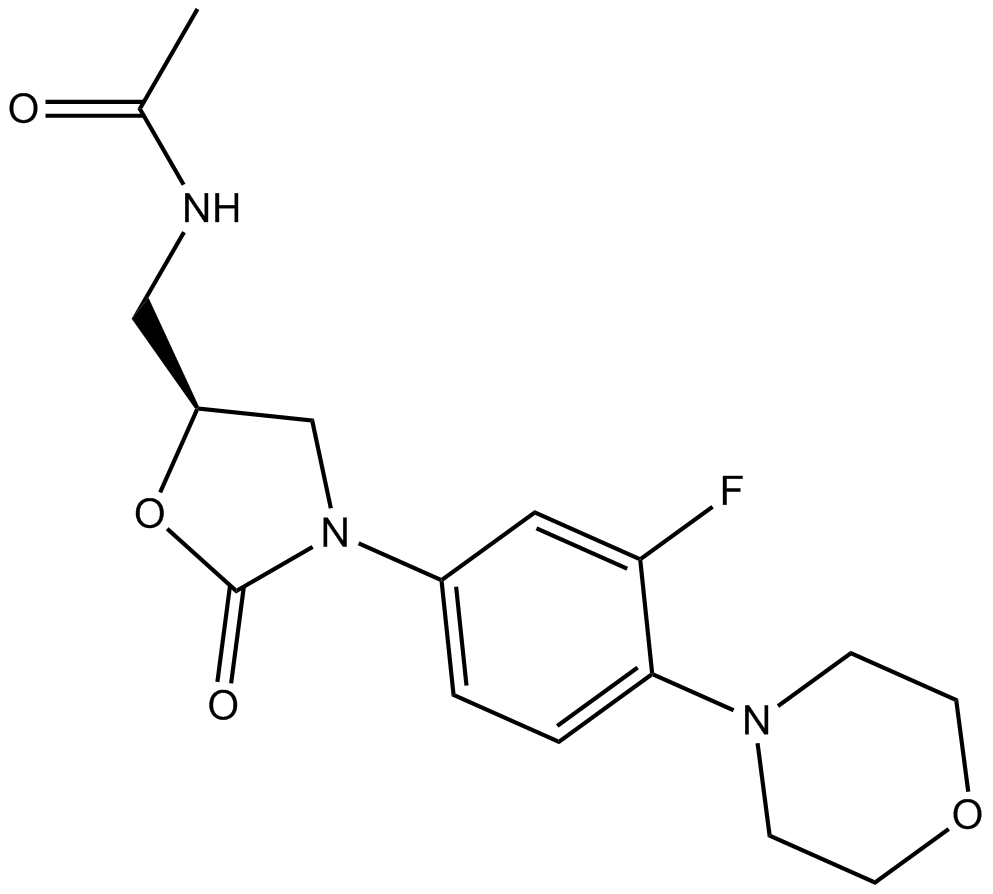
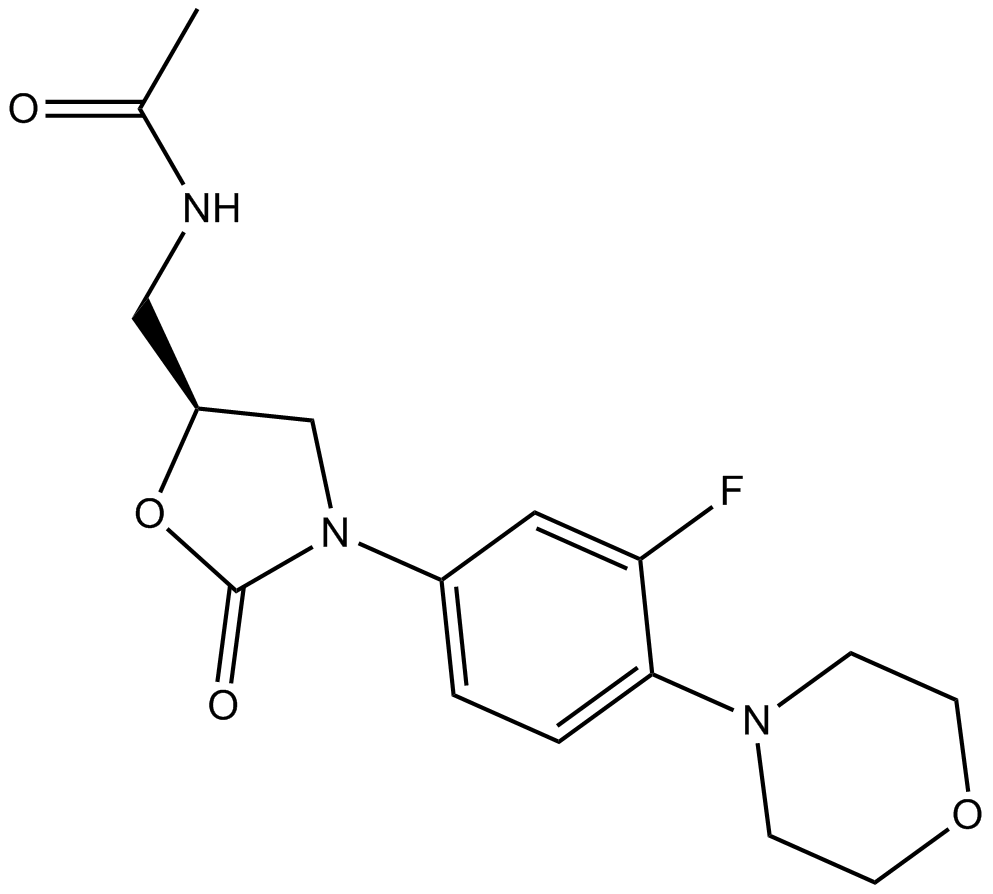
Linezolid
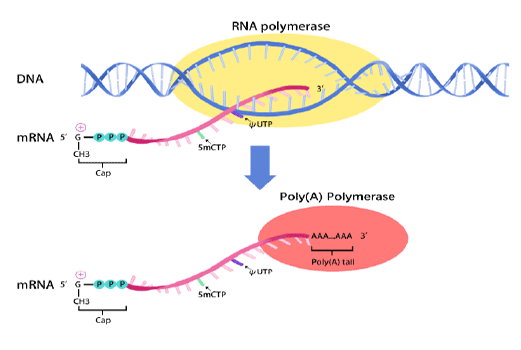
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
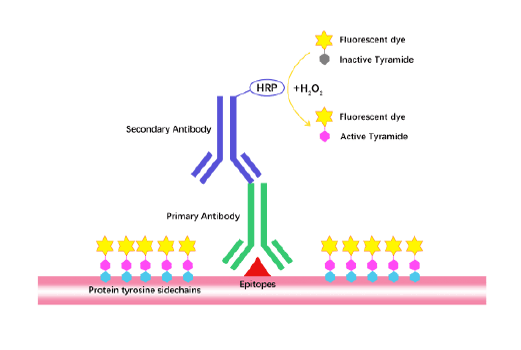
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
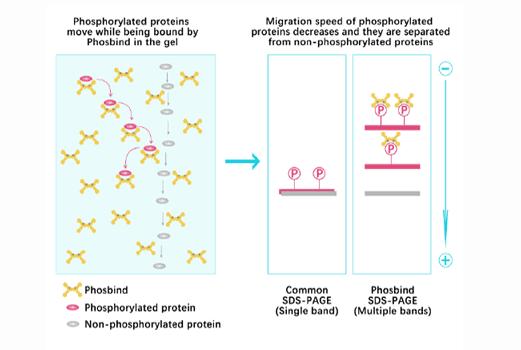
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
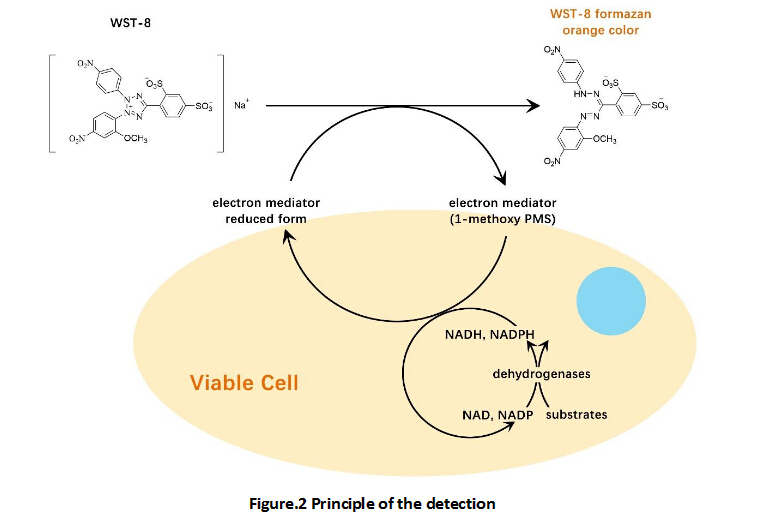
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
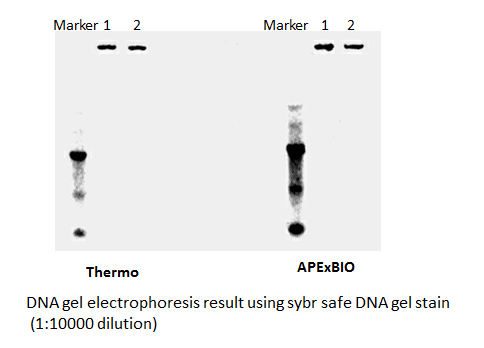
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
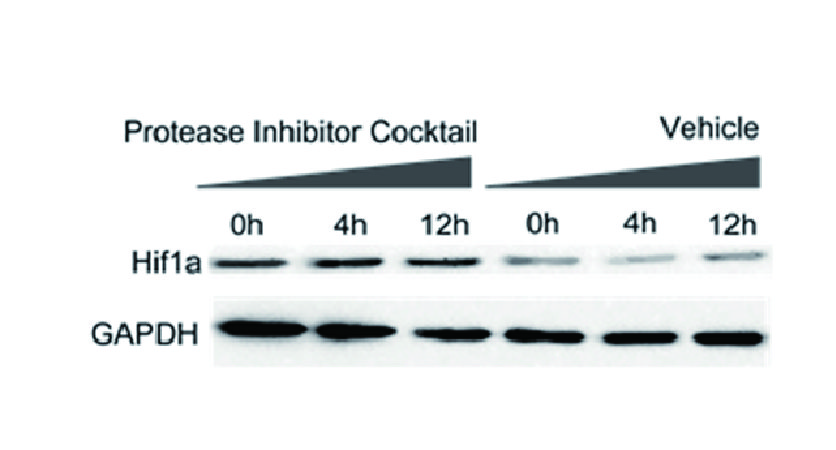
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Linezolid是合成的恶唑烷酮类抗微生物剂,其对革兰氏阳性细菌和多重耐药细菌具有广谱抗菌活性,例如链球菌、耐甲氧西林金黄色葡萄球菌(MRSA)、耐万古霉素肠球菌、耐青霉素肺炎球菌和厌氧菌[1,2]。
恶唑烷酮类可以通过结合细菌的50S亚基23S核糖体RNA上的位点抑制功能性70S-起始复合物的形成,从而抑制蛋白质合成。Linezolid是一种弱的、可逆的和非选择性的单胺氧化酶抑制剂[2]。
体外实验:Linezolid是大肠杆菌中无细胞转录翻译的有效抑制剂。IC50为1.8 mM [3]。由于测试方法以及实验室不同,测定得到的Linezolid的MIC值有所变化。对链球菌、肠球菌和葡萄球菌的MIC值在0.5和4 mg/L之间[4]。
临床试验:口服给药Linezolid具有完全生物可利用性,在口服给药后1和2小时之间达到最大的血浆浓度。Linezolid的消除半衰期为5-7小时,在治疗期间,400-600 mg linezolid每日两次给药产生稳态浓度[5]。
在皮肤/软组织感染(主要是由于金黄色葡萄球菌感染)的住院患者的临床试验中,静脉/口服Linezolid高达1250 mg/天在大于83%的个体中产生临床效果。在社区获得性肺炎患者中,成功率大于94%[6]。Linezolid也可用于治疗医院性肺炎患者[7]。Linezolid似乎具有良好的耐受性,最常见的副作用是胃肠道紊乱[6]。
参考文献:
Tsiodras S, Gold H S, Sakoulas G, et al. Linezolid resistance in a clinical isolate of Staphylococcus aureus[J]. The Lancet, 2001, 358(9277): 207-208.
Swaney S M, Aoki H, Ganoza M C, et al. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria[J]. Antimicrobial agents and chemotherapy, 1998, 42(12): 3251-3255.
Shinabarger D L, Marotti K R, Murray R W, et al. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions[J]. Antimicrobial agents and chemotherapy, 1997, 41(10): 2132-2136.
Livermore D M. Linezolid in vitro: mechanism and antibacterial spectrum[J]. Journal of antimicrobial chemotherapy, 2003, 51(suppl 2): ii9-ii16.
Stalker D J, Jungbluth G L. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial[J]. Clinical pharmacokinetics, 2003, 42(13): 1129-1140.
Clemett D, Markham A. Linezolid[J]. Drugs, 2000, 59(4): 815-827.
Wunderink R G, Cammarata S K, Oliphant T H, et al. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia[J]. Clinical therapeutics, 2003, 25(3): 980-992.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 337.35 |
| Cas No. | 165800-03-3 |
| Formula | C16H20FN3O4 |
| Solubility | ≥16.85 mg/mL in DMSO; ≥2.48 mg/mL in H2O with gentle warming and ultrasonic; ≥9.5 mg/mL in EtOH with ultrasonic |
| Chemical Name | N-[[(5S)-3-(3-fluoro-4-morpholin-4-ylphenyl)-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide |
| SDF | Download SDF |
| Canonical SMILES | CC(=O)NCC1CN(C(=O)O1)C2=CC(=C(C=C2)N3CCOCC3)F |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 抗菌实验 [1]: | |
|
细菌 |
大肠杆菌UC6782 |
|
制备方法 |
在DMSO中的溶解度大于16.9 mg/mL。若配制更高浓度的溶液,一般步骤如下:请将试管置于37 °C加热10分钟和/或将其置于超声波浴中震荡一段时间。原液于-20 °C可放置数月。 |
|
反应条件 |
0.01 ~ 1000 μM;60分钟 |
|
实验结果 |
Linezolid有效抑制大肠杆菌UC6782蛋白合成,其IC90值为30 μM。Linezolid比DuP-721的作用至少高2.5倍。Linezolid比链霉素的作用强约2倍。 |
|
References: [1]. Shinabarger D L, Marotti K R, Murray R W, et al. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions[J]. Antimicrobial agents and chemotherapy, 1997, 41(10): 2132-2136. | |
质量控制和MSDS
- 批次:
化学结构