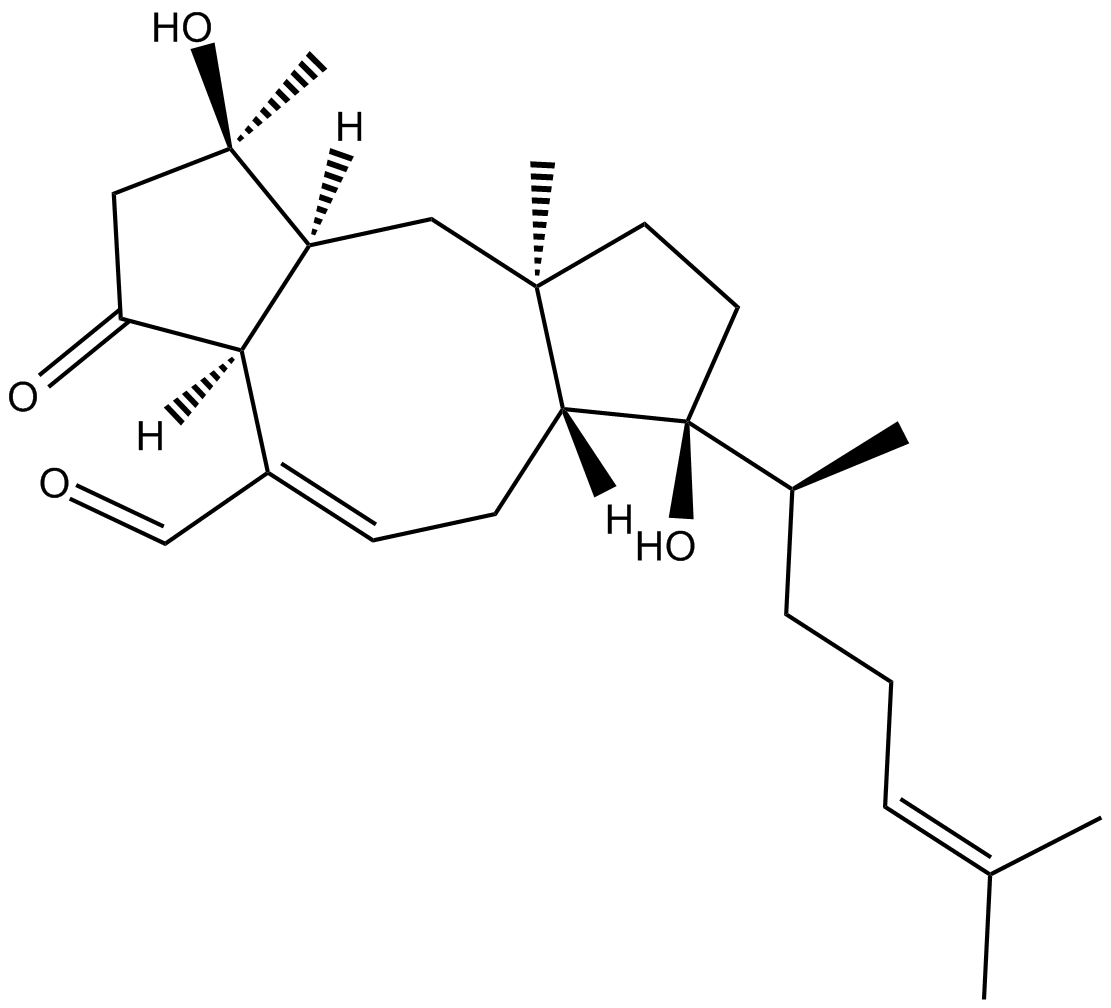
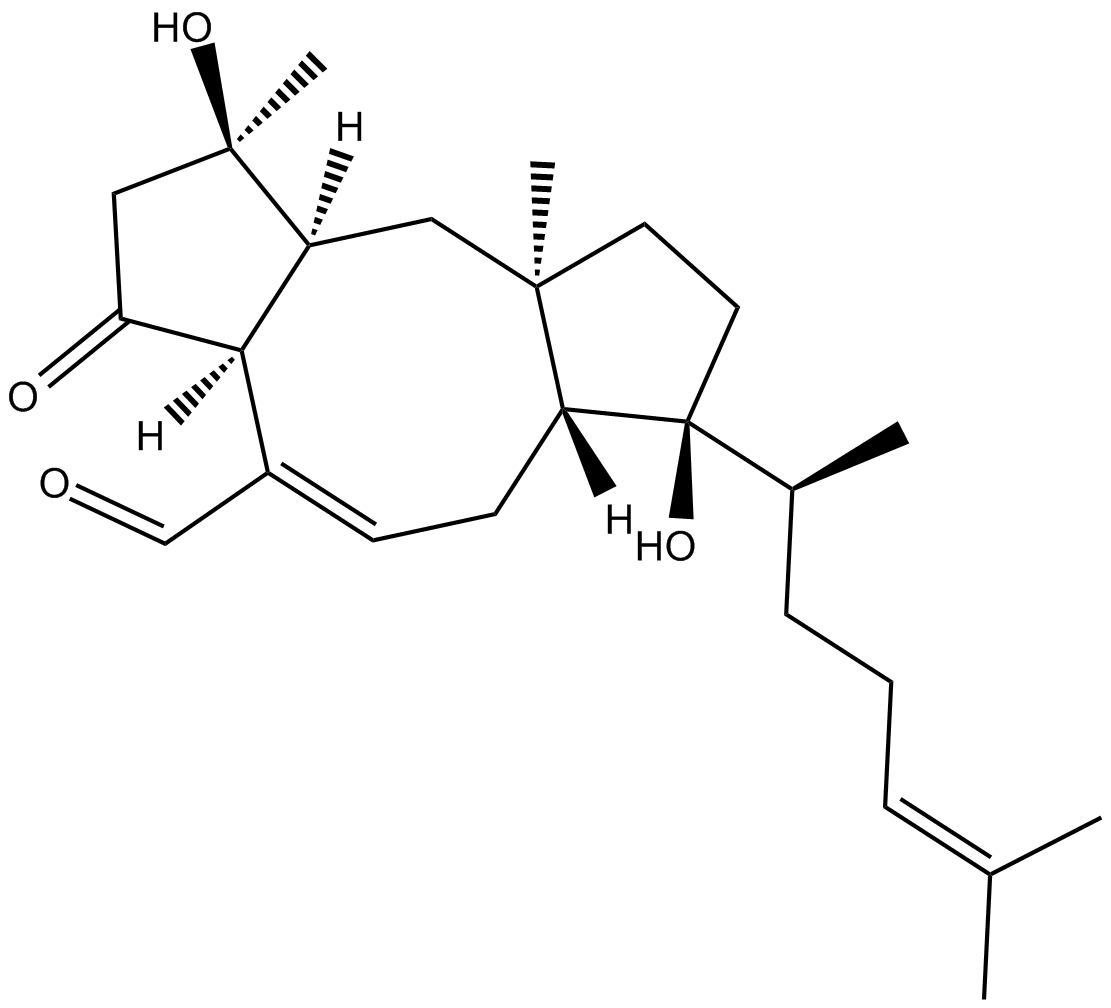
Ophiobolin B
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
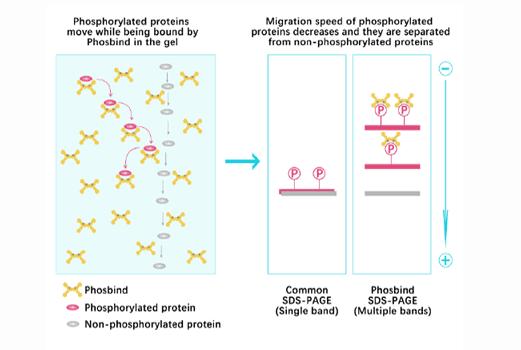
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
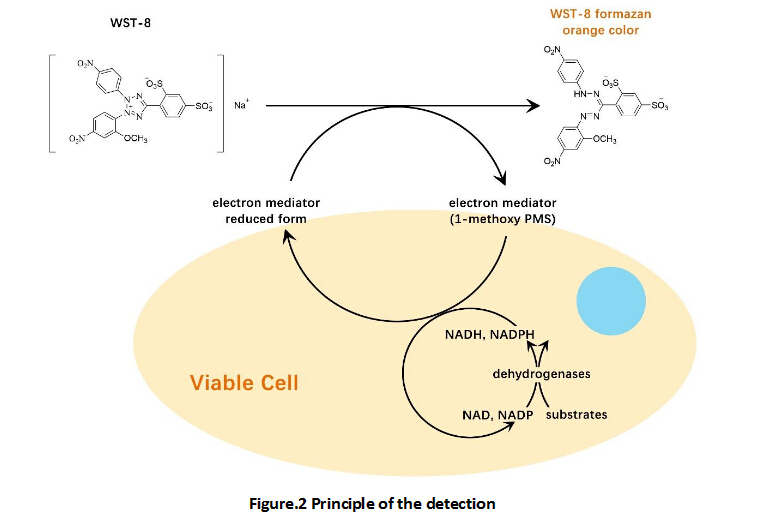
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
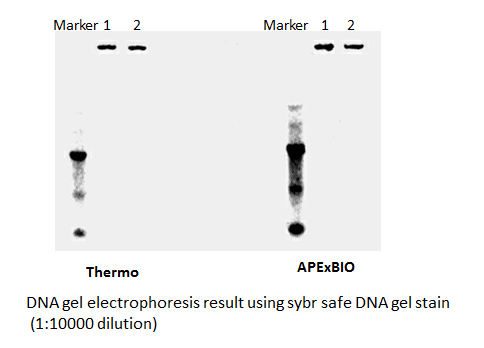
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
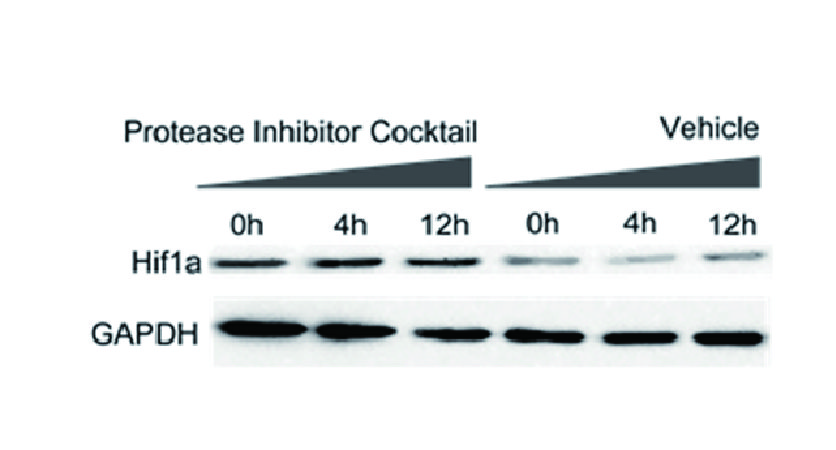
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Ophiobolins are a group of sesterterpene-type phytotoxins produced by fungi belonging to the genera Bipolaris, Drechslera, Cephalosporium and Aspergillus. Ophiobolins are secondary metabolites of fungi. To date, more than 25 ophiobolin analogues have been identified. Ophiobolins have been involved in various biological actions, such as phytotoxic, cytotoxic, nematocidal, antimicrobial and antiviral effects [1].
In vitro: In maize coleoptile tissues, Ophiobolin B inhibited proton extrusion. Ophiobolin B counteracted the biological activity of fusicoccin (FC). Ophiobolin B inhibited FC-promoted proton extrusion, potassium uptake and cell enlargement [2]. Calmodulin solutions preincubation with ophiobolin A caused an instantaneous quenching of the intrinsic tyrosine fluorescence in a time-dependent manner. The inhibitory effects of ophiobolin A could not be reversed by dialysis, dilution, nor denaturation by urea in the presence of methanol followed by renaturation. Ophiobolin A also inhibited spinach calmodulin [3]. In cultured CLL cells, treatment with increasing concentrations of Ophiobolin B for 24 h displayed bioactivity towards leukemia cells with induction of apoptosis at nanomolar concentrations [3]. Ophiobolins B exihibited antifungal effects on different zygomycetes with MIC value of 25-50 μg/ml [4].
References:
[1] Au T K, Chick W S H, Leung P C. The biology of ophiobolins[J]. Life sciences, 2000, 67(7): 733-742.
[2] Gianani L, Cocucci S, Pardi D, et al. Effects of ophiobolin B on cell enlargement and H+/K+ exchange in maize coleoptile tissues[J]. Planta, 1979, 146(3): 271-274.
[3] Bladt T T, Dürr C, Knudsen P B, et al. Bio-activity and dereplication-based discovery of ophiobolins and other fungal secondary metabolites targeting leukemia cells[J]. Molecules, 2013, 18(12): 14629-14650.
[4] Krizsán K, Bencsik O, Nyilasi I, et al. Effect of the sesterterpene-type metabolites, ophiobolins A and B, on zygomycetes fungi[J]. FEMS microbiology letters, 2010, 313(2): 135-140.
| Physical Appearance | A white solid |
| Storage | Store at -20°C |
| M.Wt | 402.6 |
| Cas No. | 5601-74-1 |
| Formula | C25H38O4 |
| Synonyms | Cochliobolin B,Ophiobolsin A,Zizanin B |
| Solubility | Soluble in DMSO |
| Chemical Name | (1R,3aS,6aR,7S,9aR,10aS)-7-[(1S)-1,5-dimethyl-4-hexen-1-yl]-1,2,3,3a,6,6a,7,8,9,9a,10,10a-dodecahydro-1,7-dihydroxy-1,9a-dimethyl-3-oxo-dicyclopenta[a,d]cyclooctene-4-carboxaldehyde |
| SDF | Download SDF |
| Canonical SMILES | C[C@@]12C[C@@]([H])([C@]3(/C(C=O)=C\C[C@]1([C@](O)(CC2)[C@H](CC/C=C(C)\C)C)[H])[H])[C@](CC3=O)(C)O |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
化学结构