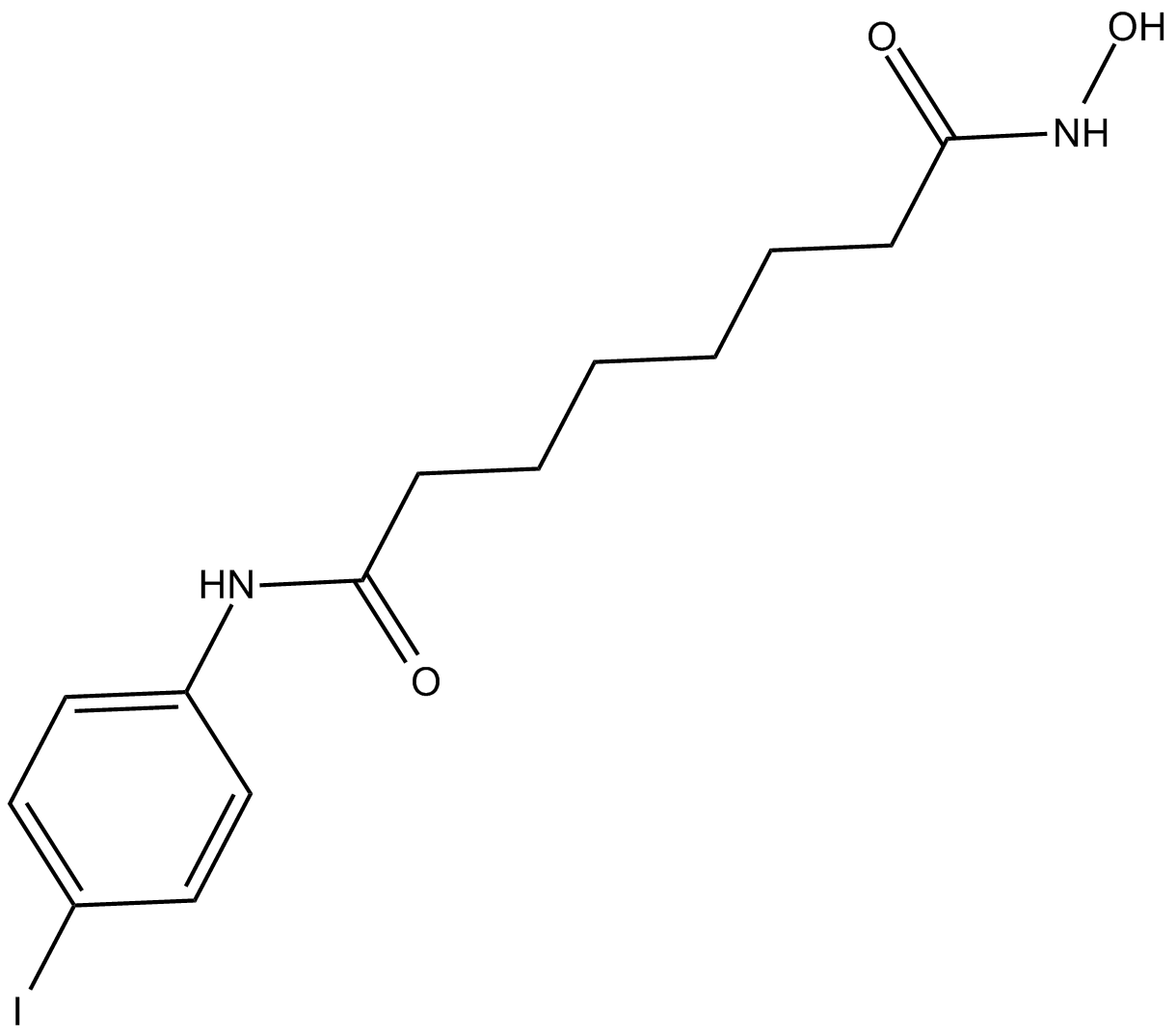
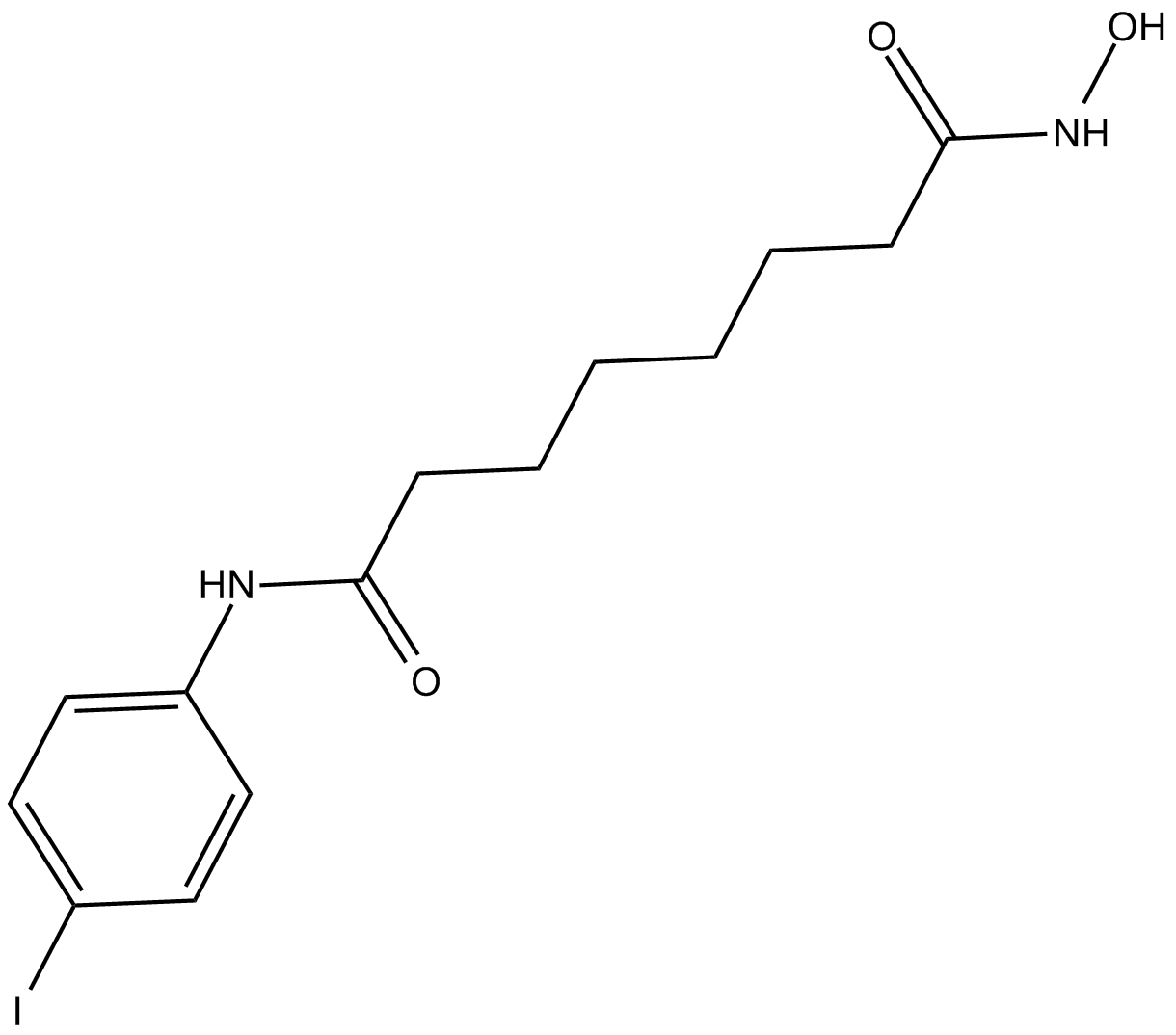
4-iodo-SAHA
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
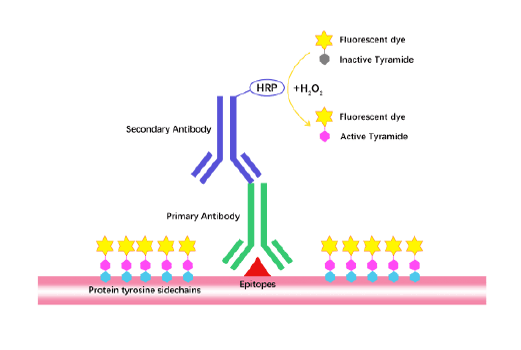
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
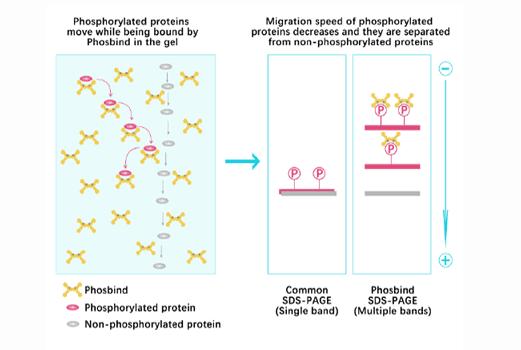
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
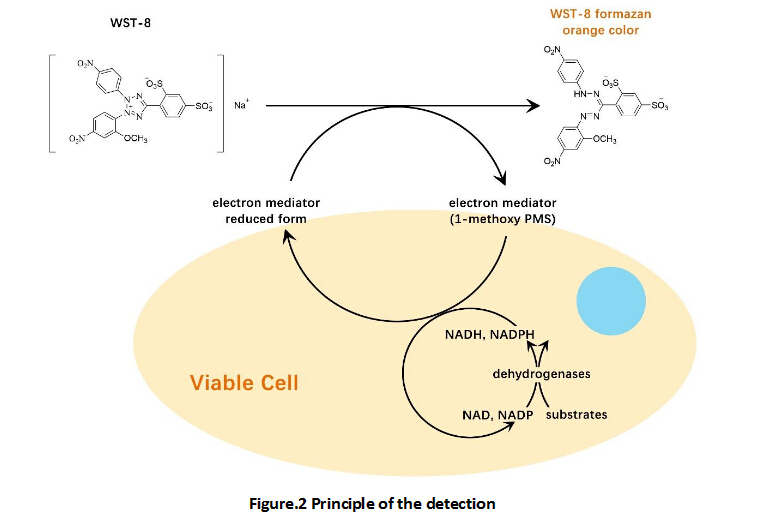
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
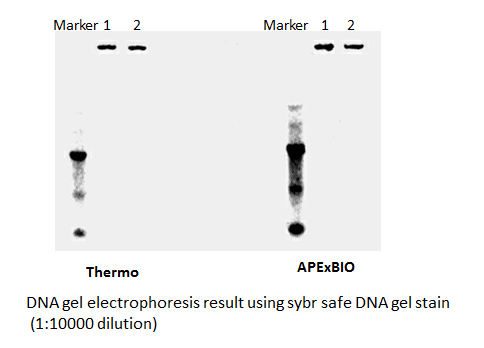
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
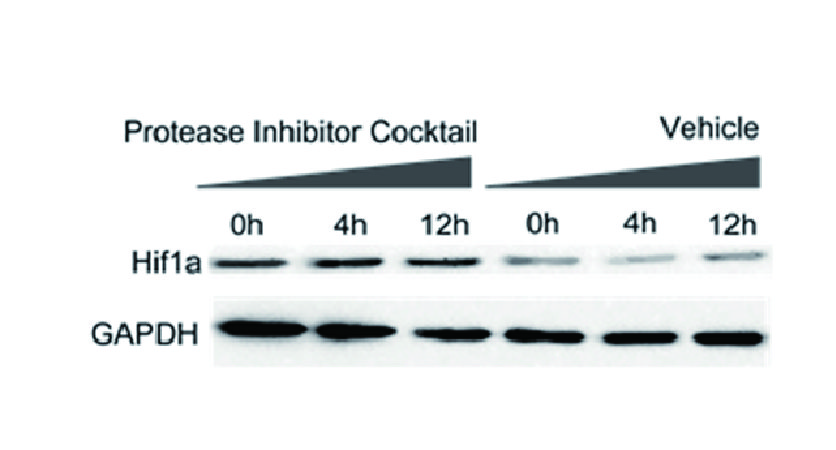
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
4-iodo-SAHA is a hydrophobic derivative of SAHA, the class I and class II histone deacetylase (HDAC) inhibitor [1].
The reversible acetylation of lysine residues in histone plays an important role in transcriptional activation and repression. The regulation of these post-translational modifications is balanced by histone acetyltransferase (HAT) and histone deacetylase (HDAC) activities. HDACs are also involved in reversible acetylation of non-histone proteins [1].
4-iodo-SAHA is a histone deacetylase (HDAC) inhibitor. In SKBR3-breast-derived cell line, 4-iodo-SAHA inhibited cell proliferation with EC50 value of 1.1 μM. In HT29 colon-derived cell line, leukemia-derived U937 tumor cell line, JA16, HL60 and K562 cell lines, 4-iodo-SAHA inhibited cell proliferation with EC50 values of 0.95, 0.12, 0.24, 0.85 and 1.3 μM, respectively. 4-iodo-SAHA is 10-fold more potent as an inhibitor of U937 leukemia cell proliferation compared to SAHA (0.12 μM versus 1.2 μM). In SKBR3 cells, 4-iodo-SAHA reduced acetylated H4 and p21 levels [1].
Reference:
[1]. Salmi-Smail C, Fabre A, Dequiedt F, et al. Modified cap group suberoylanilide hydroxamic acid histone deacetylase inhibitor derivatives reveal improved selective antileukemic activity. J Med Chem. 2010 Apr 22;53(8):3038-47.
| Storage | Store at -20°C |
| M.Wt | 390.2 |
| Cas No. | 1219807-87-0 |
| Formula | C14H19IN2O3 |
| Solubility | insoluble in H2O; insoluble in EtOH; ≥1.67 mg/mL in DMSO |
| Chemical Name | N1-hydroxy-N8-(4-iodophenyl) octanediamide |
| SDF | Download SDF |
| Canonical SMILES | IC1=CC=C(NC(CCCCCCC(NO)=O)=O)C=C1 |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
化学结构