G3335
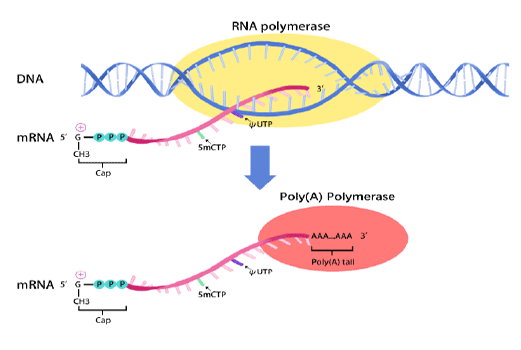
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
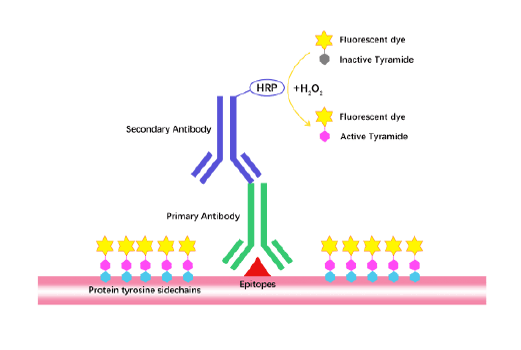
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
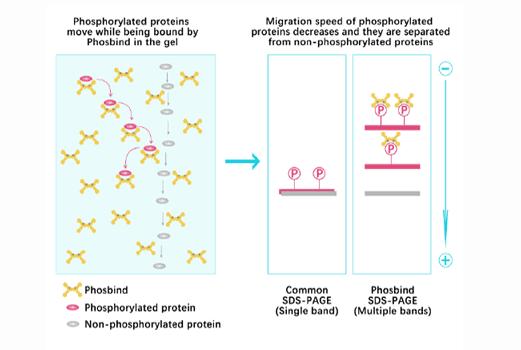
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
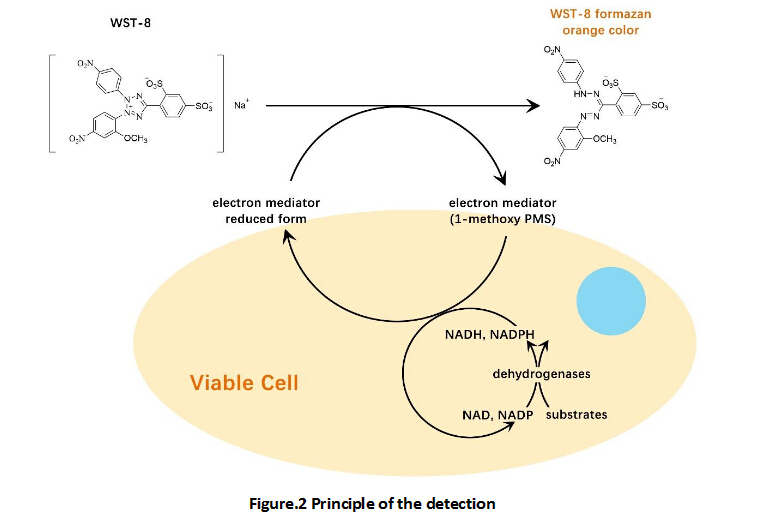
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
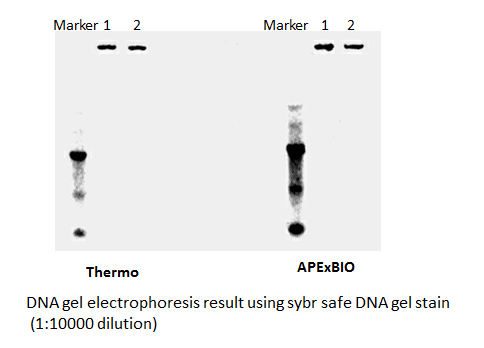
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
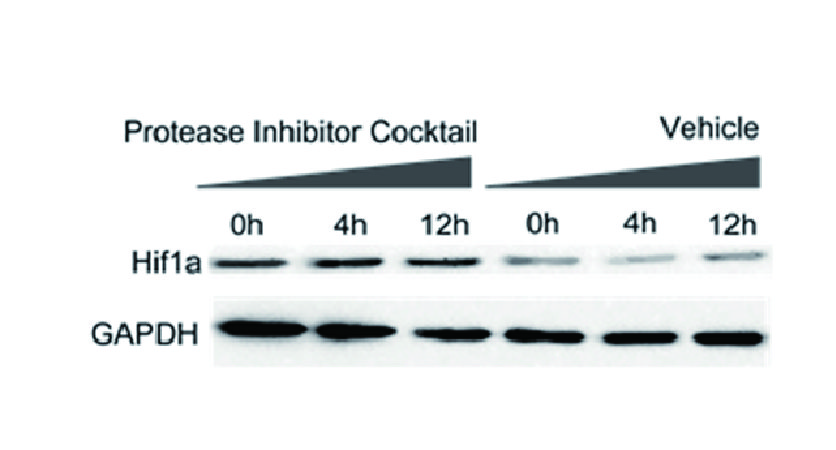
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Kd = 8.34 μM
G3335 is a PPARγ antagonist.
The peroxisome proliferator-activated receptor gamma (PPARgamma) is a key therapeutic drug target for several conditions, such as inflammation, diabetes, hypertension, dyslipidemia, and cancer.
In vitro: Biacore 3000 study based on the surface plasmon resonance technique found that G3335 exhibited a highly specific binding affinity against PPARgamma and was able to block rosiglitazone, a potent PPARgamma agonist, in the stimulation of the interaction between the PPARgamma ligand-binding domain (LBD) and RXRalpha-LBD. Moreover, the yeast two-hybrid assays indicated that G3335 had strong antagonistic activity in perturbing rosiglitazone in the promotion of the PPARgamma-LBD-CBP interaction. In addition, G3335 could competitively bind to PPARgamma against 0.1 microM rosiglitazone to repress reporter-gene expression [1].
In vivo: In a previous study, the effect of rosiglitazone was examined on spinal cord injury (SCI) in rats. The animals were randomly divided into vehicle group, rosiglitazone treated group, and G3335 treated group. Locomotor function recovery was evaluated. Results showed that compared with the vehicle groups, the rosiglitazone could significantly ameliorate locomotor recovery, reduce NF-κB expression, and increase the proliferation of endogenous NPCs. In addition, when the PPAR-γ antagonist G3335 was applied, such effects were abolished [2].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Ye, F. ,Zhang, Z.S.,Luo, H.B., et al. The dipeptide H-Trp-Glu-OH shows highly antagonistic activity against PPARγ: Bioassay with molecular modeling simulation. ChemBioChem 7, 74-82 (2006).
[2] Meng, Q. Q.,Liang, X.J.,Wang, P., et al. Rosiglitazone enhances the proliferation of neural progenitor cells and inhibits inflammation response after spinal cord injury. Neuroscience Letters 503, 191-195 (2011).
| Storage | Store at -20°C |
| M.Wt | 333.3 |
| Cas No. | 36099-95-3 |
| Formula | C16H19N3O5 |
| Solubility | insoluble in EtOH; ≥14.35 mg/mL in DMSO; ≥5.76 mg/mL in H2O with ultrasonic |
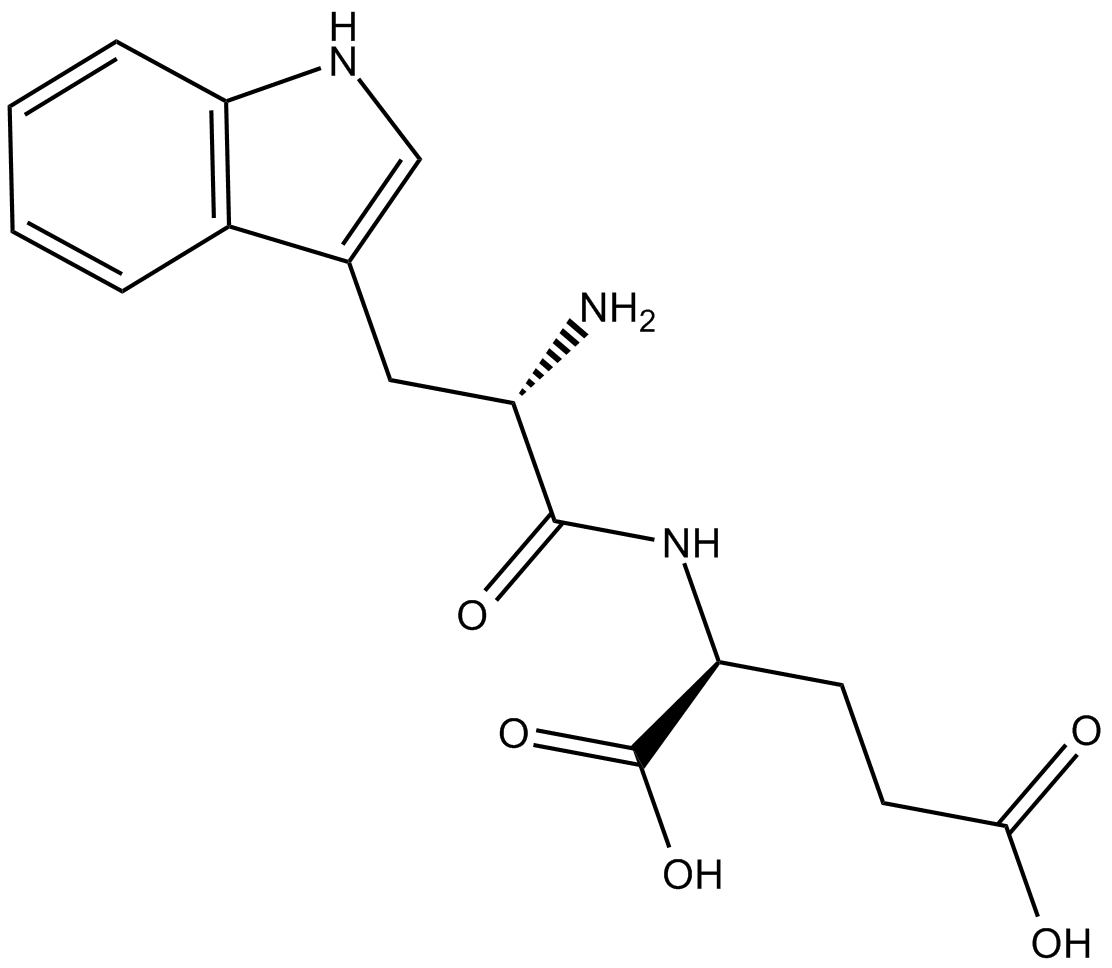
| Chemical Name | L-tryptophyl-L-glutamic acid |
| SDF | Download SDF |
| Canonical SMILES | OC(CC[C@@H](C(O)=O)NC([C@@H](N)CC1=CNC2=C1C=CC=C2)=O)=O |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |