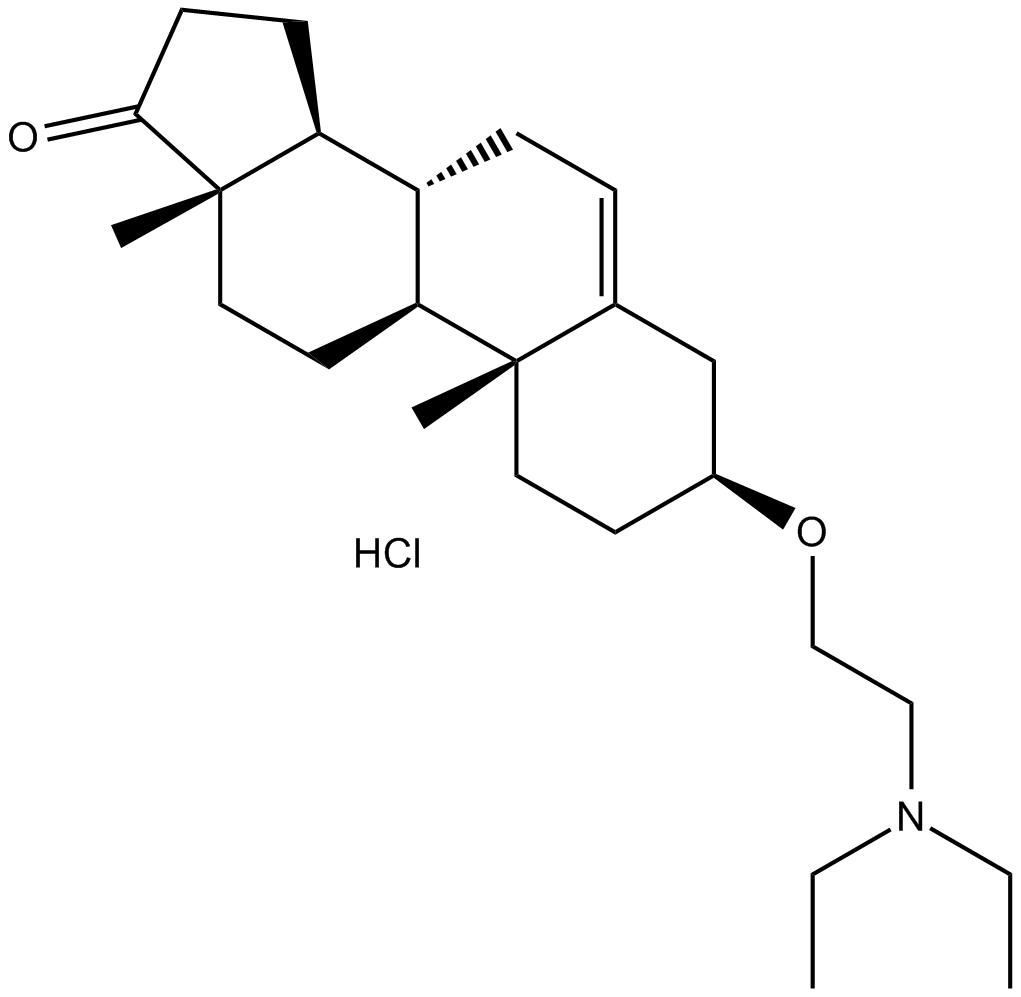
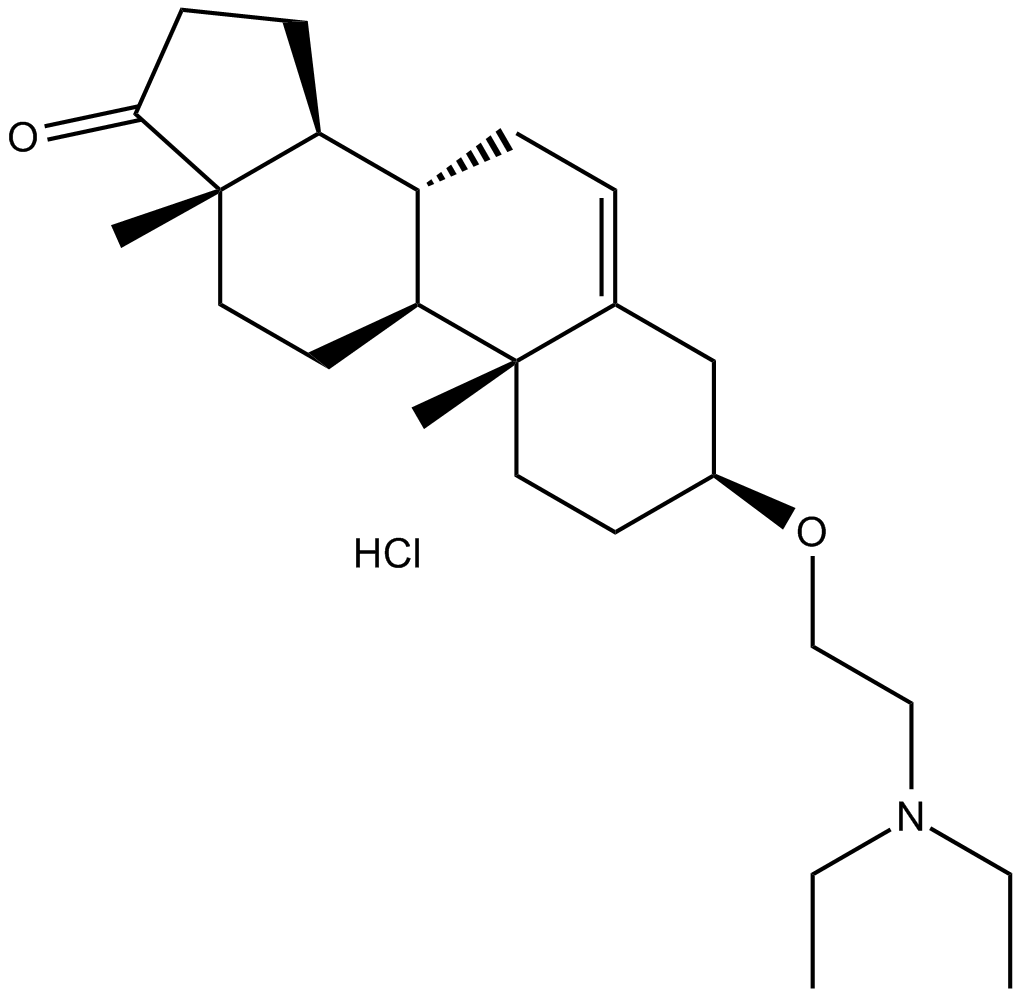
U 18666A
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
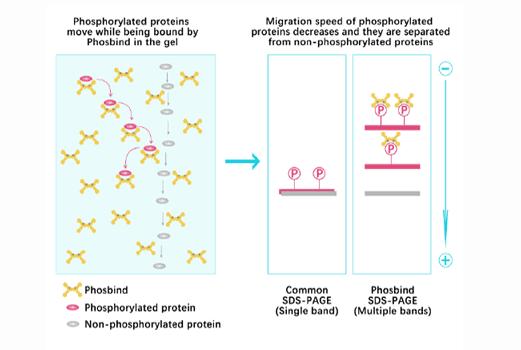
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
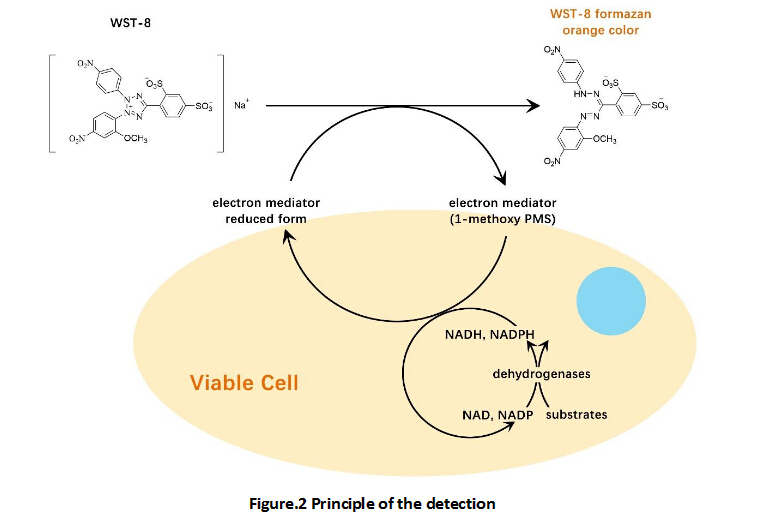
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
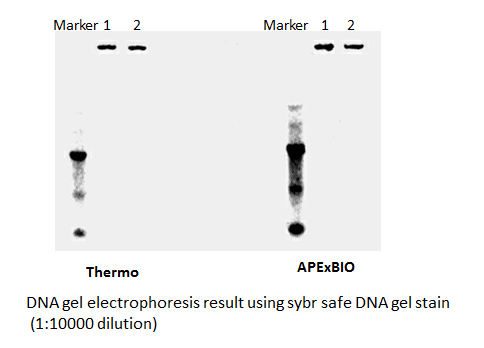
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
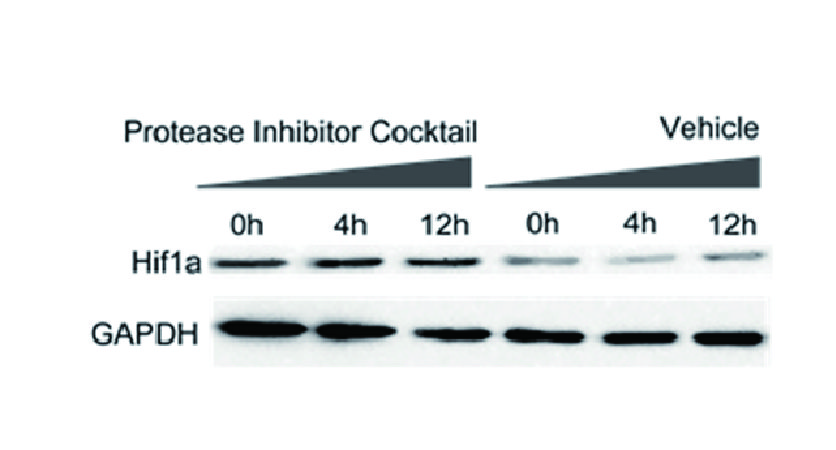
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
U 18666A is an inhibitor of cholesterol transport and synthesis [1] [2].
Cholesterol is a sterol that biosynthesized by all animal cells and is an essential component of all animal cell membranes that is required to maintain membrane fluidity and structural integrity.
U 18666A is an inhibitor of cholesterol transport and synthesis. In rat brain, U 18666A inhibited sterols production in a concentration-dependent way [1]. In cultured Chinese hamster ovary (CHO) cells, U18666A inhibited cholesterol esterification stimulated by low density lipoprotein (LDL)-derived cholesterol and also inhibited LDL receptor activities and 3-hydroxy-3-methylglutaryl-coenzyme A reductase. U18666A caused the accumulation of LDL-derived cholesterol in the lysosomes, suggesting the inhibition of LDL-derived cholesterol transport [2]. In cultured baby hamster kidney cells and human skin fibroblasts (HSF), U18666A reversibly and rapidly inhibited acyl-CoA cholesterol acyl transferase (ACAT) activated by sphingomyelinase in a dose dependent way. In sphingomyelinase-treated HSF cells, U18666A significantly and reversibly reduced the translocation of plasma membrane cholesterol. In mouse Leydig tumor cells, U18666A inhibited steroid hormones secretion stimulated by cyclic AMP in a dose dependent way [3]. In primary cortical neurons, U18666A caused cellular injury and caspase-3 activation. U18666A also caused the accumulation of cholesterol [4].
References:
[1]. Cenedella RJ. Concentration-dependent effects of AY-9944 and U18666A on sterol synthesis in brain. Variable sensitivities of metabolic steps. Biochem Pharmacol, 1980, 29(20): 2751-2754.
[2]. Liscum L, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. J Biol Chem, 1989, 264(20): 11796-11806.
[3]. Härmälä AS, Pörn MI, Mattjus P, Slotte JP. Cholesterol transport from plasma membranes to intracellular membranes is inhibited by 3 beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. Biochim Biophys Acta, 1994, 1211(3): 317-325.
[4]. Cheung NS, Koh CH, Bay BH, et al. Chronic exposure to U18666A induces apoptosis in cultured murine cortical neurons. Biochem Biophys Res Commun, 2004, 315(2): 408-417.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 424.07 |
| Cas No. | 3039-71-2 |
| Formula | C25H41NO2·HCl |
| Solubility | Soluble in H2O |
| Chemical Name | (3S,8R,9S,10R,13S,14S)-3-(2-(diethylamino)ethoxy)-10,13-dimethyl-3,4,7,8,9,10,11,12,13,14,15,16-dodecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one hydrochloride |
| SDF | Download SDF |
| Canonical SMILES | O=C1[C@]2(C)[C@@H](CC1)[C@H]3[C@H](CC2)[C@](CC[C@@H]4OCCN(CC)CC)(C)C(C4)=CC3.Cl |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 细胞实验 [1]: | |
|
细胞系 |
原发性皮层神经元 |
|
溶解方法 |
该化合物在无菌水中的溶解度为100 mM。若获取更高浓度的溶液,可在37℃下孵育10分钟,随后在超声波浴中摇匀。-20℃以下可储存数月。 |
|
反应条件 |
0.1–2.5 μg/ml,37°C,72 h |
|
应用 |
在原代培养的皮层神经元中,U18666A(2.5 μg/ml, 72 h)诱导细胞毒性。U18666A导致超过50%的细胞存活和主要形态学变化的损失,其特征在于皮质神经元中的细胞收缩和膜起泡。U18666A (0.1–2.5 μg/ml, 72 h)诱导原代培养的皮质神经元细胞损伤和细胞凋亡。 |
| 动物实验 [2]: | |
|
动物模型 |
Sprague-Dawley大鼠 |
|
给药剂量 |
皮下注射,10 mg/kg,每四或七天 |
|
应用 |
在新生大鼠中,每周注射U18666A(10 mg/kg, s.c.),连续4周,导致雄性大鼠癫痫发作阈值降低至惊厥药物氟乙基的水平。 |
|
注意事项 |
由于实验环境的不同,实际溶解度可能与理论值略有不同,请测试室内所有化合物的溶解度。 |
|
References: [1]. Cheung N S, Koh C H V, Bay B H, et al. Chronic exposure to U18666A induces apoptosis in cultured murine cortical neurons[J]. Biochemical and biophysical research communications, 2004, 315(2): 408-417. [2]. Bierkamper G G, Cenedella R J. Induction of chronic epileptiform activity in the rat by an inhibitor of cholesterol synthesis, U18666A[J]. Brain research, 1978, 150(2): 343-351. | |
化学结构