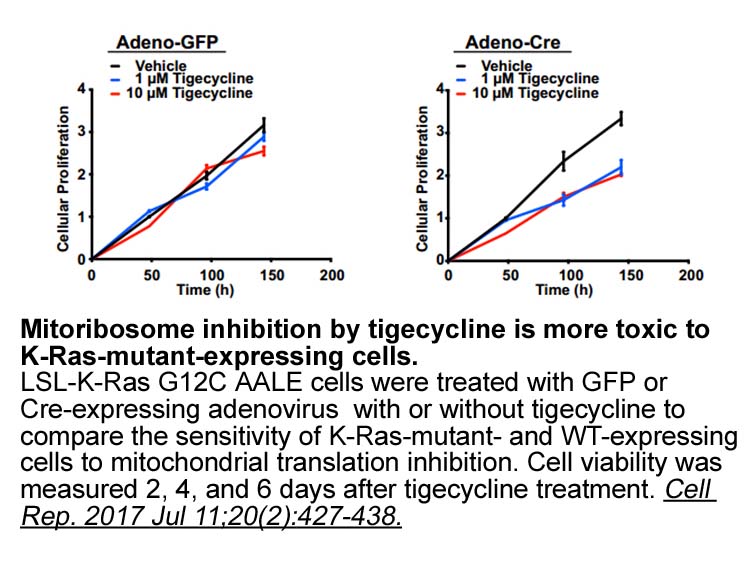
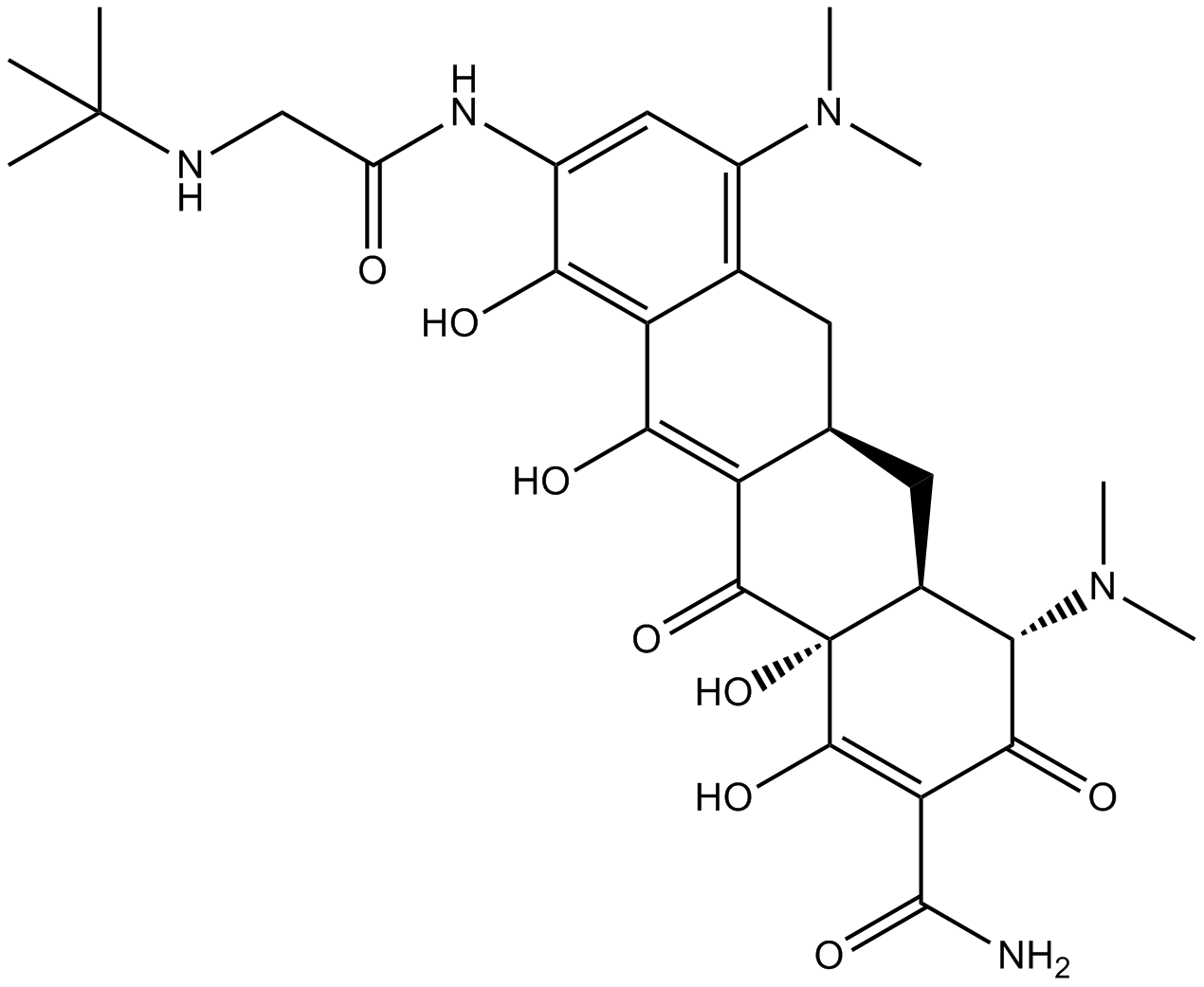
Tigecycline
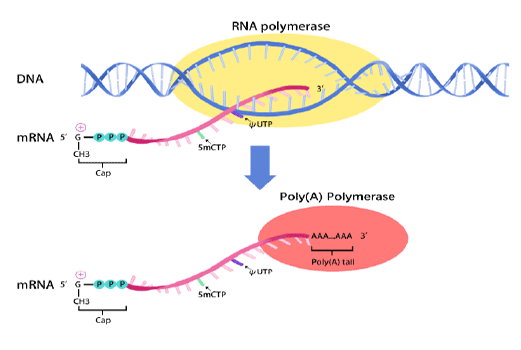
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
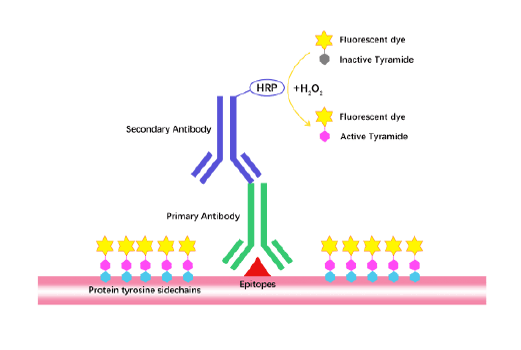
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
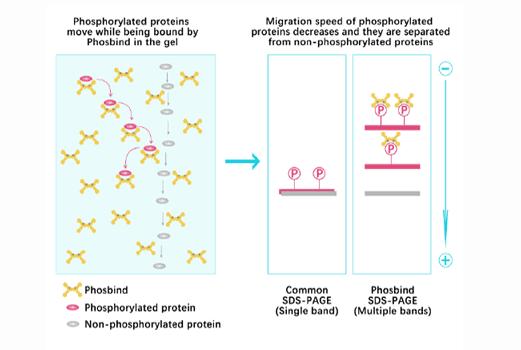
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
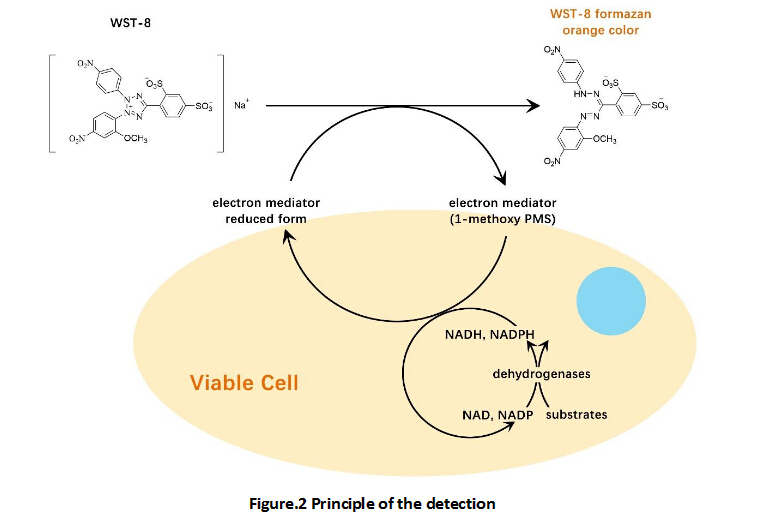
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
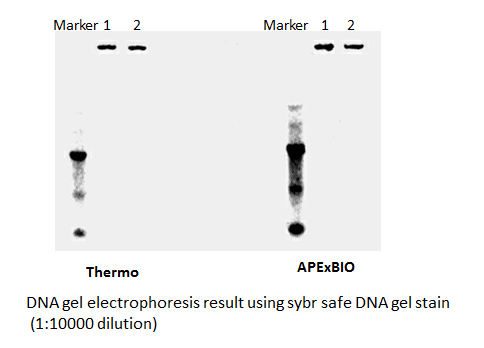
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
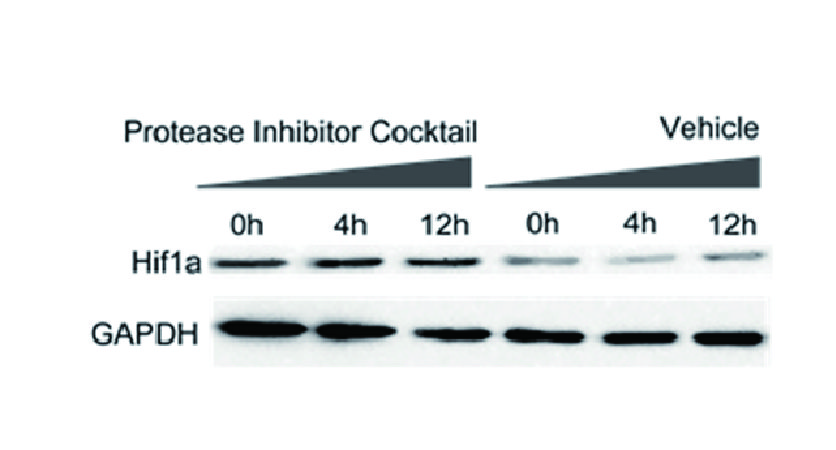
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Tigecycline是第一个商业化的甘氨酰环素成员,是一类新的抗微生物剂。
甘氨酰环素是对四环素抗生素进行结构修饰后得到的衍生物,对革兰氏阳性、革兰氏阴性和厌氧活性菌有效,包括某些多药耐药菌株[1]。Tigecycline通过可逆地结合30S核糖体亚基和抑制蛋白质翻译而抑制细菌[1]。
体外实验:Tigecycline具有良好的体外活性,对肠炎肠球菌和屎肠球菌的万古霉素敏感和抗性菌株的MIC90范围为0.12-0.5 μg/ml [2]。Tigecycline在细胞中浓缩并主要通过胆汁排泄消除。肾功能的减少没有显著改变其系统清除率。Tigecycline不干扰常见的细胞色素P450酶,使得药代动力学药物相互作用不常见[3]。Tigecycline的组织渗透性非常好,该化合物在腹内感染中与亚胺培南/西司他汀效果相当,在皮肤和皮肤结构感染中与万古霉素和氨曲南共同作用效果相当[4]。
在体实验:在腹膜内系统性小鼠感染模型中,证明了Tigecycline针对GISA、甲氧西林抗性金黄色葡萄球菌和甲氧西林敏感金黄色葡萄球菌菌株具有体内效力[2]。在由MSSA菌株GC 4543引起的感染中,Tigecycline和daptomycin具有相似的体内效力,ED50分别为0.12和0.24 mg/kg。Tigecycline的ED50为0.72 mg/kg [2]。
临床试验:对于皮肤和皮肤结构感染的住院患者,50 mg剂量的Tigecycline给药12h,临床治愈率和微生物根除率分别为74%和70%,接受25-mg剂量的患者中为67%和56% [5]。副作用表现为恶心和呕吐。Tigecycline单独给药治疗与万古霉素-氨曲南组合治疗一样安全有效[6]。
参考文献:
Rose W E, Rybak M J. Tigecycline: first of a new class of antimicrobial agents[J]. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 2006, 26(8): 1099-1110.
Petersen P J, Bradford P A, Weiss W J, et al. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens[J]. Antimicrobial agents and chemotherapy, 2002, 46(8): 2595-2601.
Stein G E, Craig W A. Tigecycline: a critical analysis[J]. Clinical infectious diseases, 2006, 43(4): 518-524.
Livermore D M. Tigecycline: what is it, and where should it be used[J]. Journal of Antimicrobial Chemotherapy, 2005, 56(4): 611-614.
Postier R G, Green S L, Klein S R, et al. Results of a multicenter, randomized, open-label efficacy and safety study of two doses of tigecycline for complicated skin and skin-structure infections in hospitalized patients[J]. Clinical therapeutics, 2004, 26(5): 704-714.
Grosse E J E, Babinchak T, Dartois N, et al. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam[J]. Clinical infectious diseases, 2005, 41(Supplement 5): S341-S353.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 585.65 |
| Cas No. | 220620-09-7 |
| Formula | C29H39N5O8 |
| Solubility | ≥29.3 mg/mL in DMSO; insoluble in EtOH; ≥32.47 mg/mL in H2O with ultrasonic |
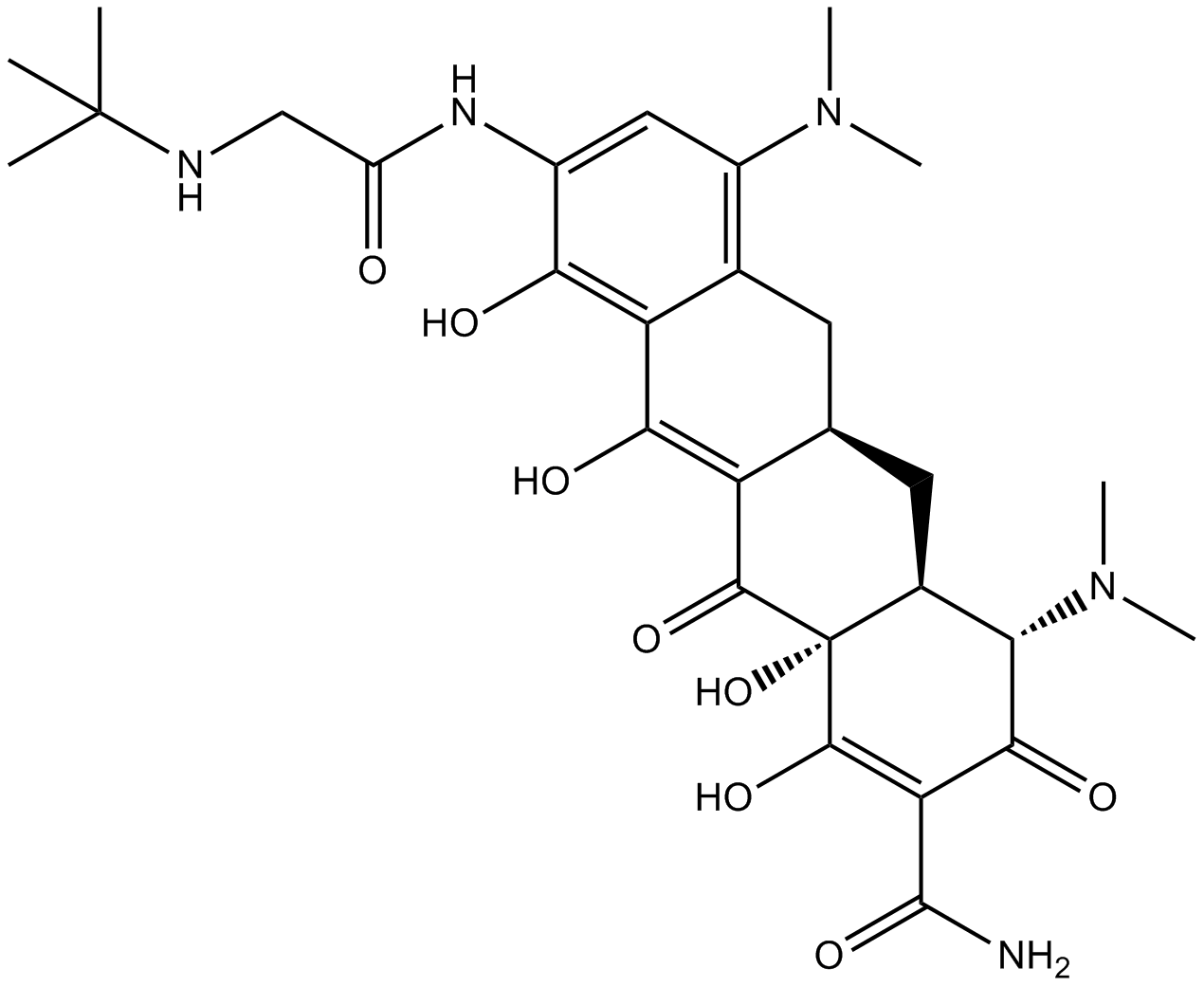
| Chemical Name | (4S,4aS,5aR,12aR)-9-[[2-(tert-butylamino)acetyl]amino]-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide |
| SDF | Download SDF |
| Canonical SMILES | CN(C)C1=C2C(C(O)=C(C([C@@](C(O)=C3C(N)=O)(O)[C@@H]4[C@H](N(C)C)C3=O)=O)[C@H](C4)C2)=C(O)C(NC(CNC(C)(C)C)=O)=C1 |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 抗菌药敏试验 [1]: | |
|
细菌 |
耐药以及敏感革兰氏阳性菌株 |
|
制备方法 |
在DMSO中的溶解度大于29.3 mg/mL。若配制更高浓度的溶液,一般步骤如下:请将试管置于37 °C加热10分钟和/或将其置于超声波浴中震荡一段时间。原液于-20 °C可放置数月。 |
|
反应条件 |
18 ~ 22小时 |
|
实验结果 |
Tigecycline对GISA葡萄球菌、耐Methicillin葡萄球菌和Methicillin敏感葡萄球菌显示出类似的体外活性 (MIC90 = 0.5 ~ 1 μg/mL)。此外,Tigecycline还对Vancomycin敏感的和耐Vancomycin的粪肠球菌和屎肠球菌显示出良好的体外活性,其MIC90值为0.12 ~ 0.5 μg/mL。 |
| 动物实验 [1]: | |
|
动物模型 |
通过腹腔注射细菌制作的小鼠全身感染模型 |
|
给药剂量 |
0.2 mL,0.01 M;静脉注射;单剂量 |
|
实验结果 |
对于由MSSA菌株引起的感染,Daptomycin和Tigecycline显示出类似的体内作用,其ED50值分别为0.12 mg/kg和0.24 mg/kg。此外,Tigecycline和Daptomycin对于由MRSA菌株引起的感染也显示出类似的体内作用,其ED50值分别为0.72 mg/kg和0.87 mg/kg。然而,Tigecycline对由GISA菌株引起的感染的作用最大,其ED50值为1.9 mg/kg,比Daptomycin (ED50 = 6.1 mg/kg) 的作用高3倍多。 |
|
注意事项 |
请于室内测试所有化合物的溶解度。虽然化合物的实际溶解度可能与其理论值略有不同,但仍处于实验系统误差的允许范围内。 |
|
References: [1]. Petersen P J, Bradford P A, Weiss W J, et al. In vitro and in vivo activities of tigecycline (GAR-936), daptomycin, and comparative antimicrobial agents against glycopeptide-intermediate Staphylococcus aureus and other resistant gram-positive pathogens[J]. Antimicrobial agents and chemotherapy, 2002, 46(8): 2595-2601. |
|
质量控制和MSDS
- 批次:
化学结构
相关生物数据