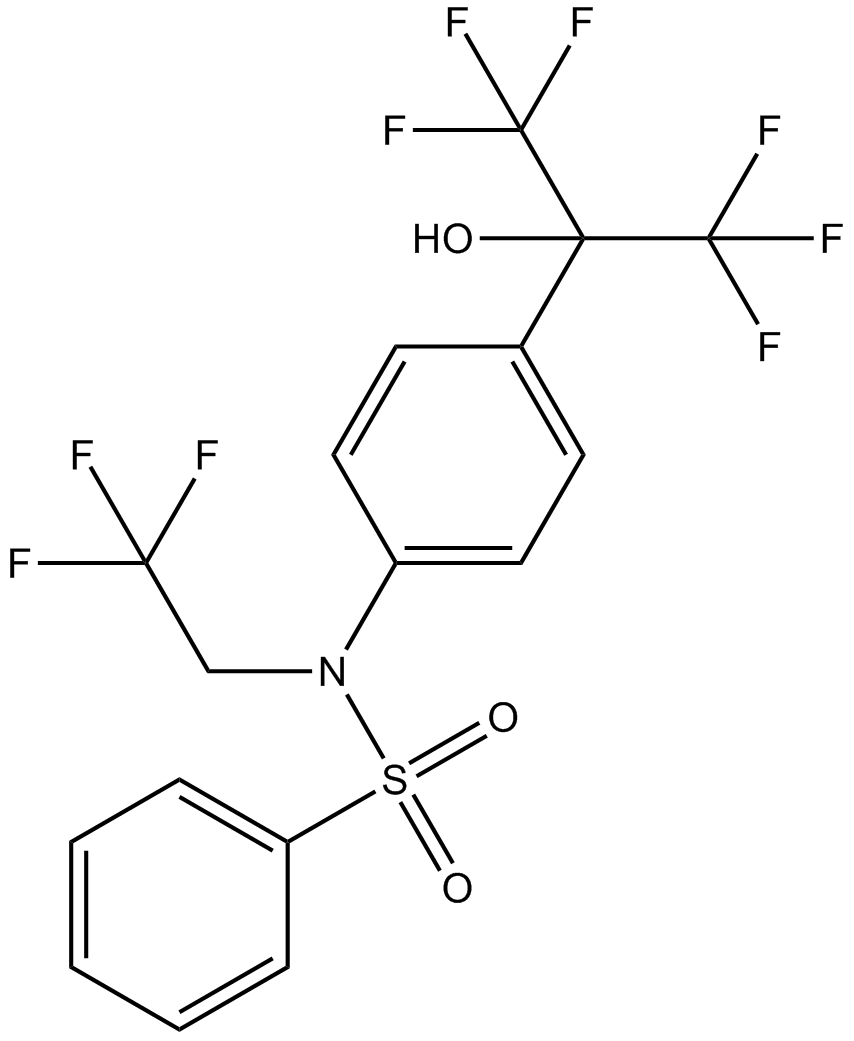
T0901317
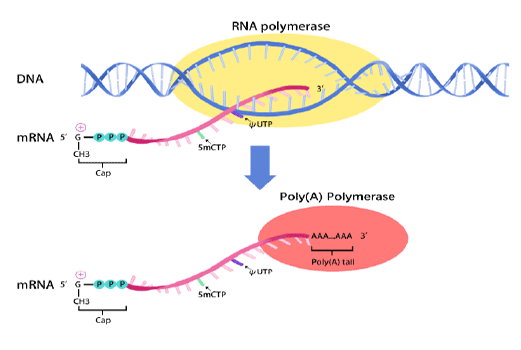
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
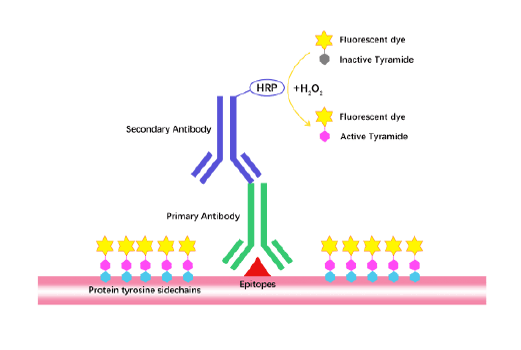
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
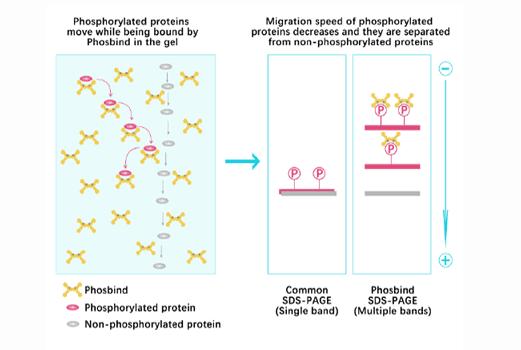
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
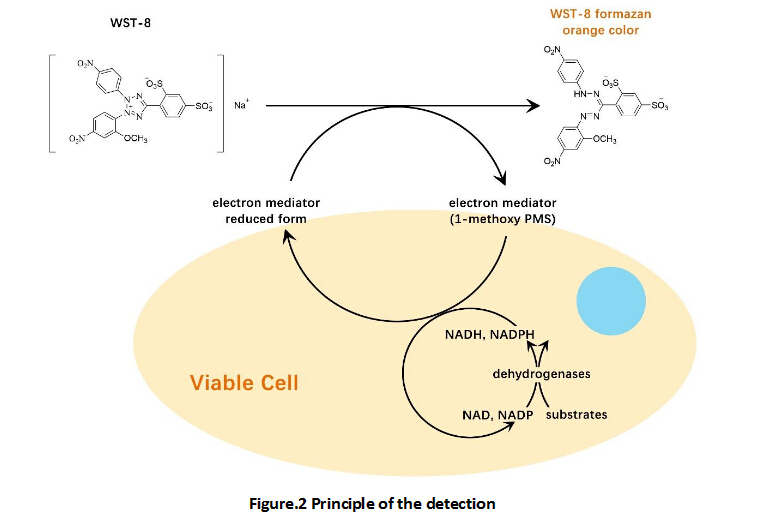
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
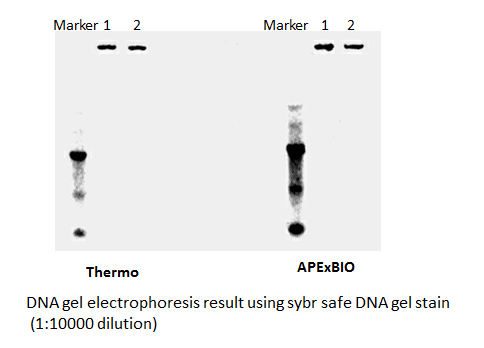
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
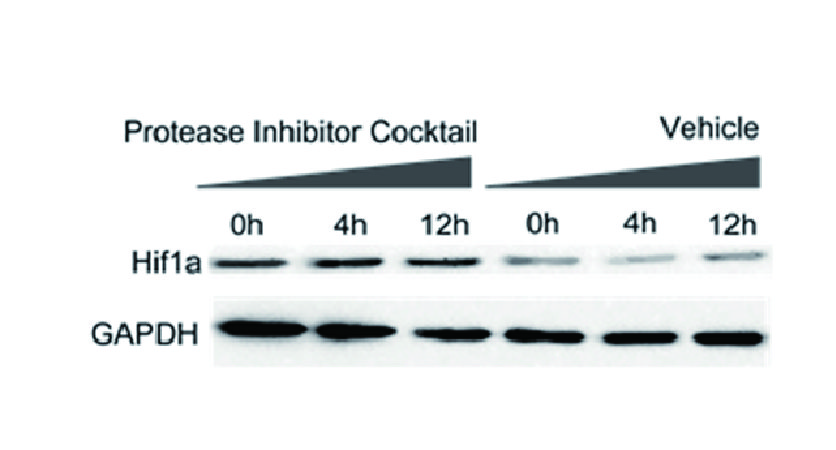
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
T0901317是多靶点激动剂,作用于LXRα和FXR,EC50值分别为20 nM和5 μM。而且,T0901317也是RORα和RORγ的反向激动剂,IC50值分别为2.0 μM和1.7 μM[1,2]。
LXRs在胆固醇代谢中起重要作用,是调控几个重要脂质代谢的核受体,包括胆固醇和胆汁酸。LXRs与FXR最初均被认为是核受体超级组的孤儿成员。而FXR后来被鉴定为胆汁酸的生理受体,可调节胆汁酸转运与合成的反馈回路,还可调节脂代谢中的附加功能。视黄酸受体相关孤儿受体(RORs)在多种生理过程中起重要作用,包括肝脏糖异生、脂代谢、昼夜节律及免疫功能。T0901317通过高亲和性的直接结合,抑制RORα和RORγ的转录激活活性,从而调控受体与转录辅因子相互作用的能力,而对RORβ没有抑制活性[1,2]。
罗丹明标记肽用于T0901317与LXRα结合的荧光偏振检测。在该同质生化试验中,罗丹明标记肽与LXR结合程度越高,观察到的荧光偏振程度就越强。测定T0901317与LXRα结合的效力,EC50值为20 nM。在共转染Gal4 DBD-FXR配体结合域嵌合受体和Gal4反应性荧光素酶报告基因载体的HEK293细胞中,T0901317激活FXR,EC50值约为5μM,超过了天然FXR配体的效力。在基于细胞的GAL4-NR LBD共转染实验中,T0901317在2 μM时有效抑制GAL4-RORα和GAL4-RORγ。而且,T0901317抑制GAL4-RORα和GAL4-RORγ的组成型转录激活活性,而对GAL4-RORβ很少或没有活性,证明了T0901317的选择性[1、2、3]。
研究T0901317对ABCA1表达的体内作用。在11周龄的APP23小鼠中,T0901317以50 mg/kg/day的剂量,以口服灌胃的方式给药6天后显著增加ABCA1的表达,而APP的表达没有变化[4]。
参考文献:
1. Schultz J R, Tu H, Luk A, et al. Role of LXRs in control of lipogenesis[J]. Genes & development, 2000, 14(22): 2831-2838.
2. Kumar N, Solt L A, Conkright J J, et al. The benzenesulfoamide T0901317 [N-(2, 2, 2-trifluoroethyl)-N-[4-[2, 2, 2-trifluoro-1-hydroxy-1-(trifluoromethyl) ethyl] phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-α/γ inverse agonist[J]. Molecular pharmacology, 2010, 77(2): 228-236.
3. Houck K A, Borchert K M, Hepler C D, et al. T0901317 is a dual LXR/FXR agonist[J]. Molecular genetics and metabolism, 2004, 83(1): 184-187.
4. Liu Y, Yan C, Wang Y, et al. Liver X receptor agonist T0901317 inhibition of glucocorticoid receptor expression in hepatocytes may contribute to the amelioration of diabetic syndrome in db/db mice[J]. Endocrinology, 2006, 147(11): 5061-5068.
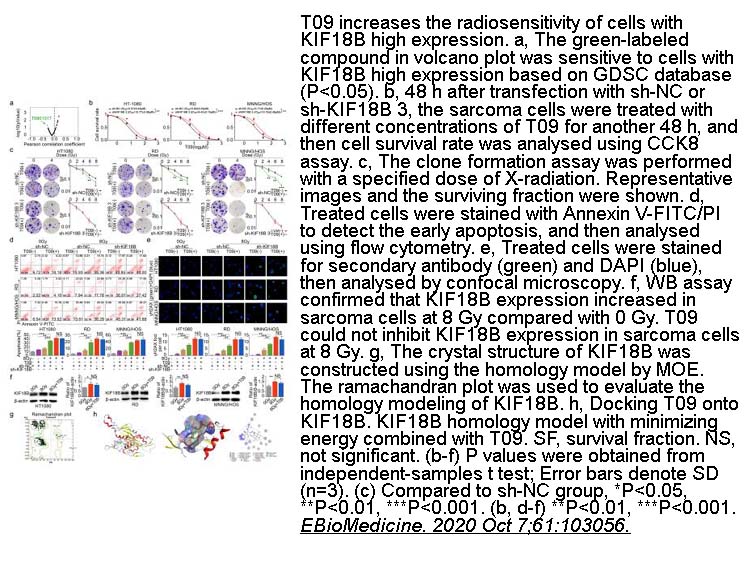
- 1. Wensi Liu, Zhaojin Yu, et al. "Silencing KIF18B enhances radiosensitivity: identification of a promising therapeutic target in sarcoma." EBioMedicine. 2020 Oct 7;61:103056. PMID:33038765
- 2. Wang X, Cai B, et al. "Cholesterol Stabilizes TAZ in Hepatocytes to Promote Experimental Non-alcoholic Steatohepatitis." Cell Metab. 2020;31(5):969-986.e7. PMID:32259482
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 481.33 |
| Cas No. | 293754-55-9 |
| Formula | C17H12F9NO3S |
| Solubility | ≥24.05 mg/mL in DMSO; insoluble in H2O; ≥55.6 mg/mL in EtOH |
| Chemical Name | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide |
| SDF | Download SDF |
| Canonical SMILES | C1=CC=C(C=C1)S(=O)(=O)N(CC(F)(F)F)C2=CC=C(C=C2)C(C(F)(F)F)(C(F)(F)F)O |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 激酶实验[1]: | |
|
结合实验 |
将LXR配体结合结构域与谷胱甘肽S-转移酶(GST)的C末端融合,并将所得的GST-LXR蛋白在大肠杆菌中表达并于谷胱甘肽珠上纯化。将10 nM罗丹明标记的肽(ILRKLLQE)在振荡器上与400 nM GST-LXR和T0901317溶于100 μL缓冲液(10 mMHepes、150 mMNaCl、2mM MgCl2、5mM DTT、pH7.9)的96孔板中,在LJL分析器(LJL Biosystems)上测量荧光偏振(mP)。 |
| 细胞实验 [1]: | |
|
细胞系 |
转染表达人LXRα质粒的HEK293细胞。 |
|
溶解方法 |
该化合物在DMSO中的溶解度大于10 mM。若配制更高浓度的溶液,一般步骤如下:请将试管置于37℃加热10分钟和/或将其置于超声波浴中震荡一段时间。原液于-20℃可放置数月 |
|
反应条件 |
20 h. |
|
实验结果 |
T0901317是一种高效和选择性非甾体类的LXR配体。T0901317诱导接近8倍的LXRα的转录活性,EC50值为20 nM。T0901317还反式激活嵌合体Gal4-LXRα和Gal4-PXR(孕烷X受体)。 |
| 动物实验 [1]: | |
|
动物模型 |
6至10周龄C57BL/6小鼠; 12至16周龄的金色叙利亚仓鼠。 |
|
剂量 |
小鼠: 5、50 mg/kg,口服;仓鼠: 3、10、30 mg/kg,口服. |
|
实验结果 |
口服T0901317的C57BL/6小鼠血浆中甘油三酯的水平显著增加。在仓鼠中,T0901317也增加血浆甘油三酯。此外,T0901317增加脂肪酸代谢相关基因和肝脂肪酸生物合成基因(ACC (2倍), FAS (3倍)和SCD-1 (9倍))的表达。 |
|
注意事项 |
请测试所有化合物在室内的溶解度,实际溶解度和理论值可能略有不同。这是由实验系统的误差引起的,属于正常现象。 |
|
References: [1]. Schultz JR, Tu H, Luk A, et al. Role of LXRs in control of lipogenesis. Genes Dev, 2000, 14(22): 2831-2838. |
|
| 描述 | T0901317是一种有效的和选择性的LXRα和LXRβ激动剂,Kd值分别为7 nM和22 nM。 | |||||
| 靶点 | LXRα | LXRβ | ||||
| IC50 | 7 nM | 22 nM | ||||
质量控制和MSDS
- 批次:
化学结构
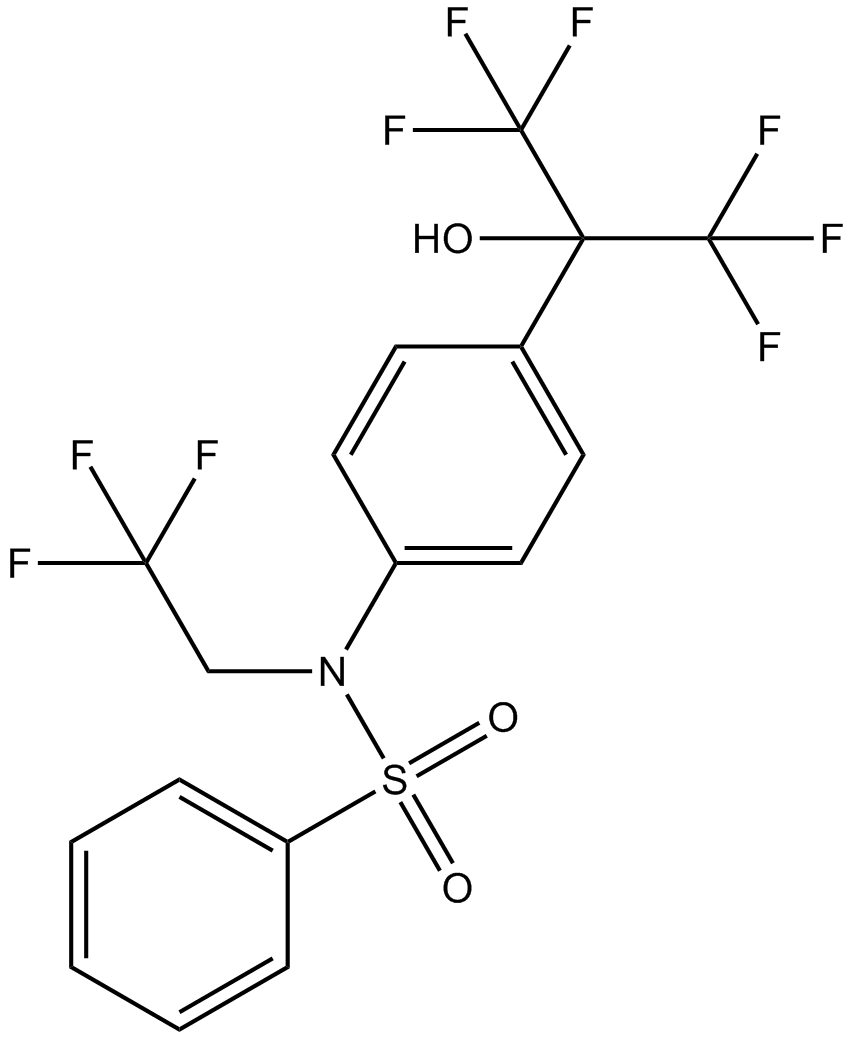
相关生物数据