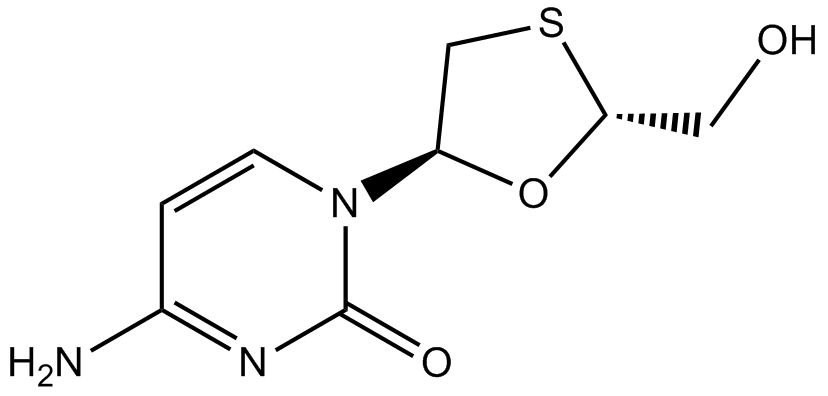
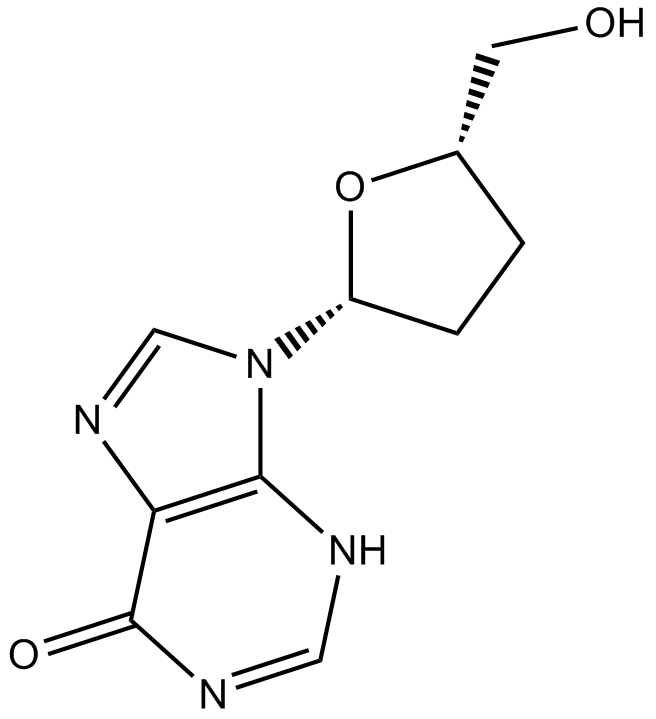
Lamivudine
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
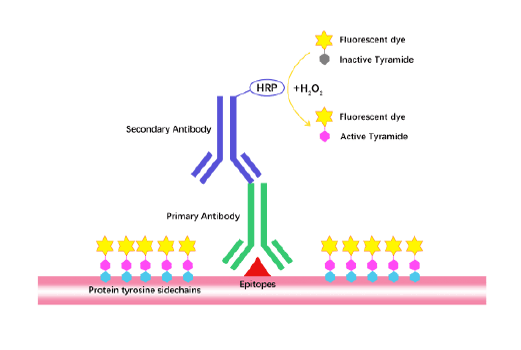
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
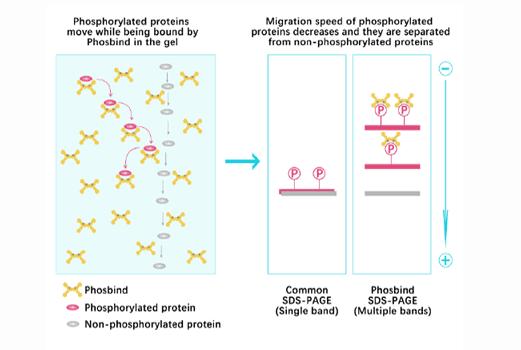
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
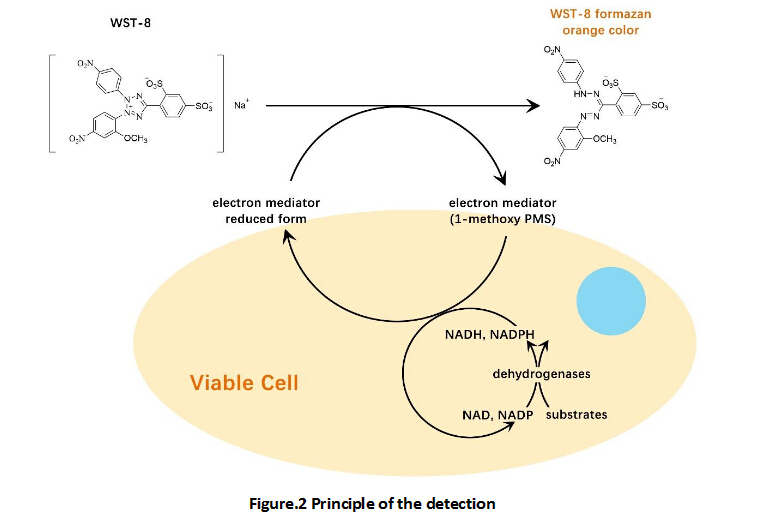
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
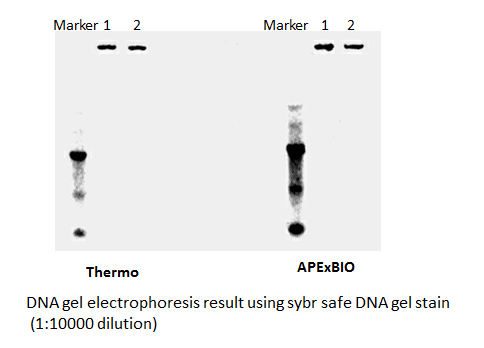
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
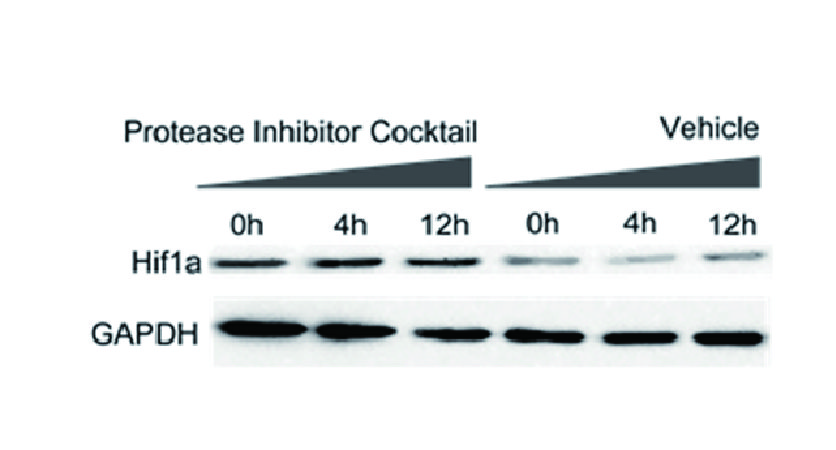
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Lamivudine, a nucleoside analog, is a potent reverse transcriptase inhibitor, with IC50 value of 0.316 µM against human immunodeficiency virus type 1 (HIV-1) [1].
Reverse transcriptase is a RNA-dependent DNA polymerase, playing a critical role in retroviral replication. Thus, reverse transcriptase has been made a target of antiretroviral chemotherapy [1].
Lamivudine was most potent against simian retrovirus types 1 and 2 (SRV-1, SRV-2), and HIV-1, with IC50 values of 10, 0.316 and 0.316 µM, respectively, but did not inhibit foamy viruses and amphotrophic murine leukaemia virus (MLV-A) [1].
In human coinfected with HIV-1 and hepatitis B virus (HBV), Lamivudine (150 mg, b.i.d., p.o.) displayed dual efficacy through both delayed HIV-1 disease progression and a favorable biochemical and HBV virologic response. Twelve months of Lamivudine therapy led to HBV DNA and hepatitis B e antigen (HBeAg) losses of ∼40% and ∼20%, respectively, and more alanine aminotransferase (ALT) normalization than that in the placebo group [2].
References:
[1]. Rosenblum L L, Patton G, Grigg A R, et al. Differential susceptibility of retroviruses to nucleoside analogues. Antiviral Chemistry and Chemotherapy, 2001, 12(2): 91-97.
[2]. Dore G J, Cooper D A, Barrett C, et al. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. The Journal of Infectious Diseases, 1999, 180(3): 607-613.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 229.26 |
| Cas No. | 134678-17-4 |
| Formula | C8H11N3O3S |
| Solubility | ≥10.5 mg/mL in DMSO; ≥11.4 mg/mL in EtOH with ultrasonic; ≥89.4 mg/mL in H2O with ultrasonic |
| Chemical Name | 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one |
| SDF | Download SDF |
| Canonical SMILES | C1C(OC(S1)CO)N2C=CC(=NC2=O)N |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| Cell experiment:[1] | |
|
Cell lines |
Peripheral blood mononuclear cells (PBMCs) |
|
Reaction Conditions |
10 d incubation |
|
Applications |
Lamivudine inhibited p24 antigen production by HIV-I in PBMCs, with ED50s ranging from 0.07 to 0.2 μM. There was no toxicity in uninfected PBMC with concentrations of lamivudine up to 10 μM over a 10-day culture period. |
| Animal experiment:[2] | |
|
Animal models |
Humanized BALB/c Rag1-/-γc-/- or Rag2-/-γc-/- mice |
|
Dosage form |
7 mg drug tablet containing lamivudine, abacavir and dolutegravir Re-suspended in 100 μl distilled water for daily gavage |
|
Applications |
Oral drug treatment led to full viral suppression and protection from CD4 T cell depletion. Cessation of therapy resulted in viral rebound and CD4 T cell loss. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Merrill DP, Moonis M, Chou TC, et al. Lamivudine or stavudine in two- and three-drug combinations against human immunodeficiency virus type 1 replication in vitro. The Journal of Infectious Diseases, 1996, 173(2): 355-364. 2. Hu S, Neff CP, Kumar DM, et al. A humanized mouse model for HIV-2 infection and efficacy testing of a single-pill triple-drug combination anti-retroviral therapy. Virology, 2017, 501: 115-118. |
|
质量控制和MSDS
- 批次:
化学结构