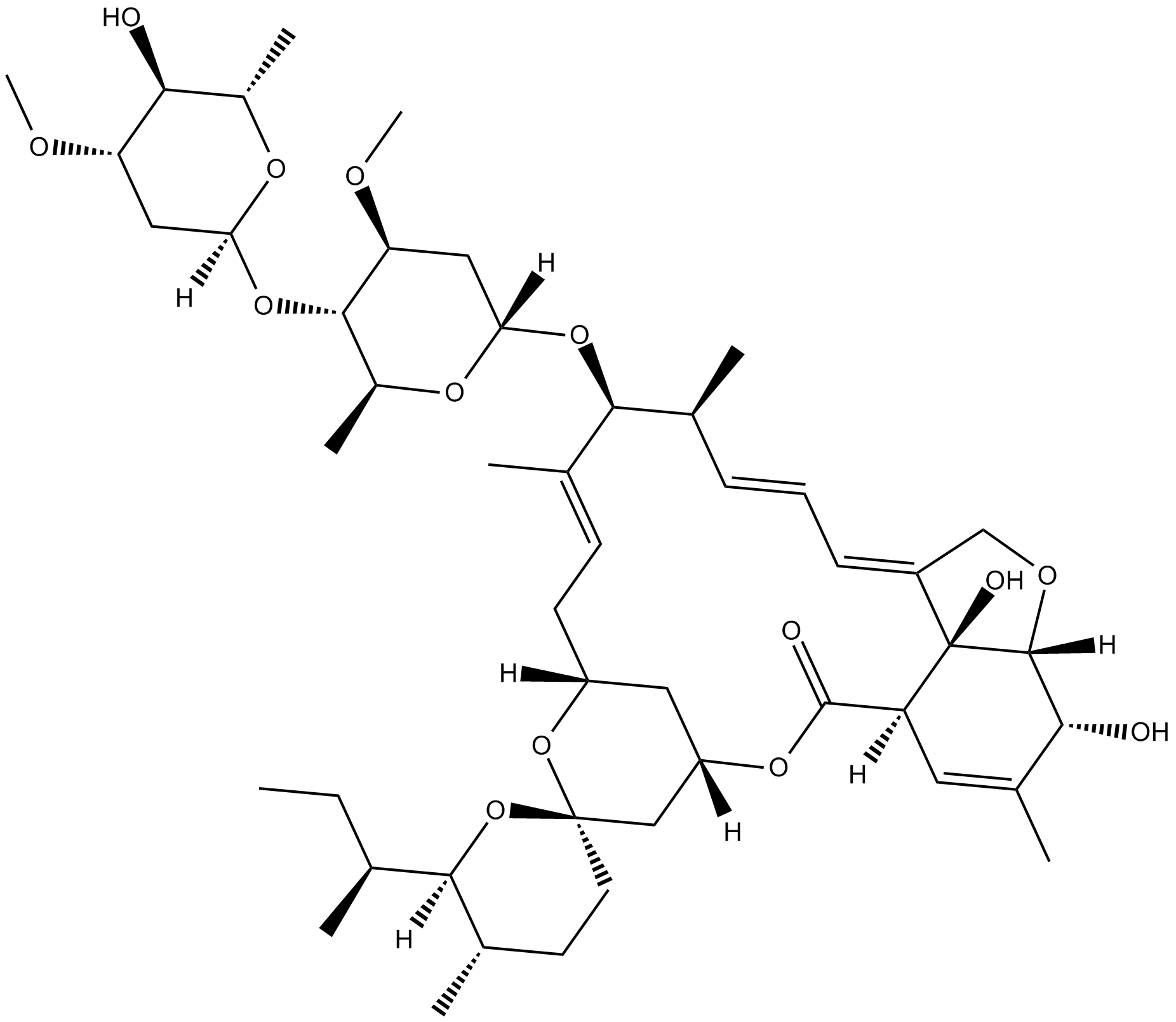
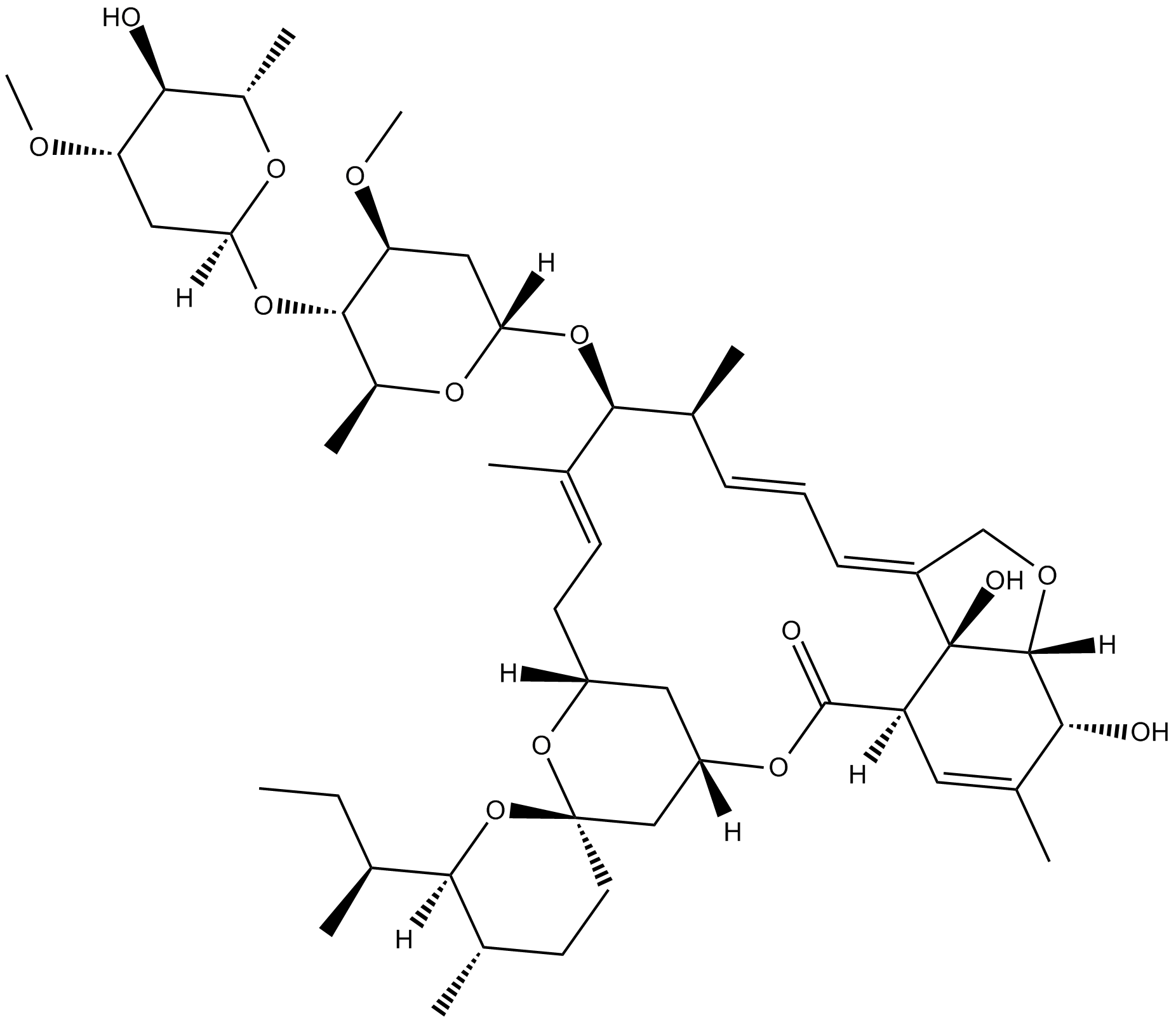
Ivermectin B1a
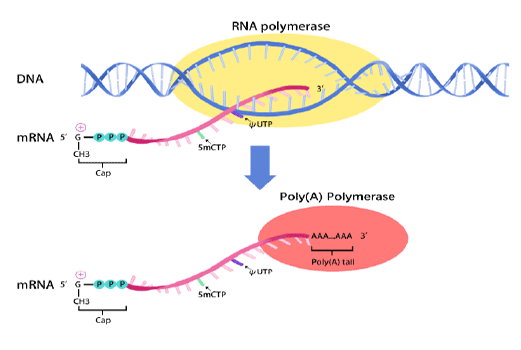
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
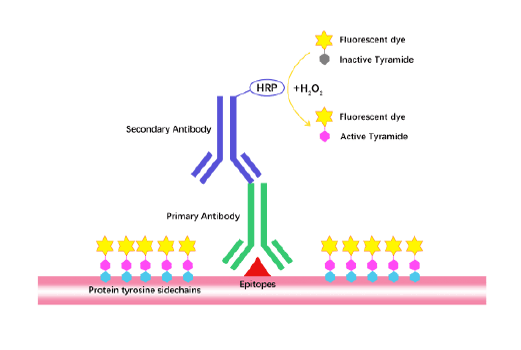
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
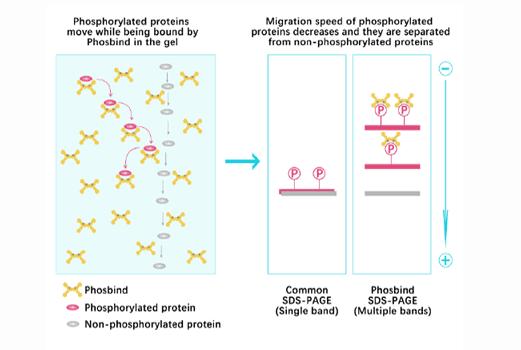
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
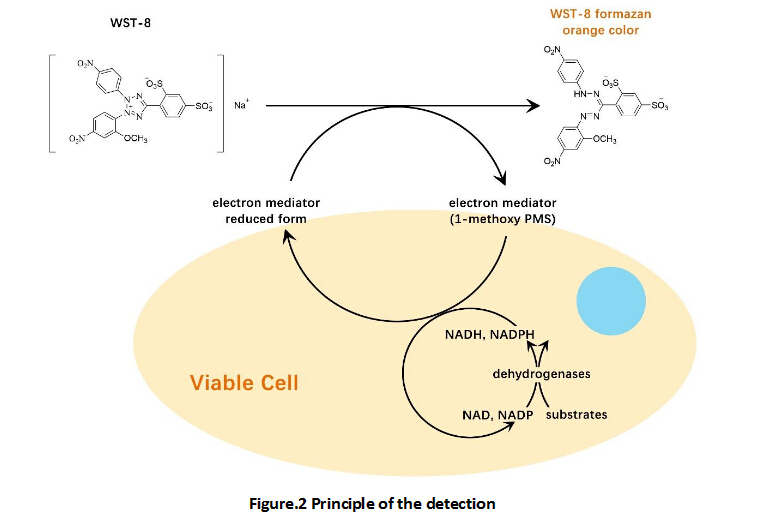
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
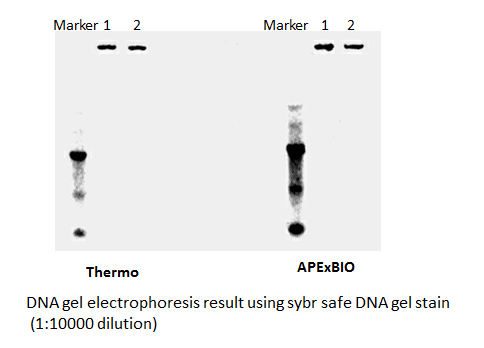
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
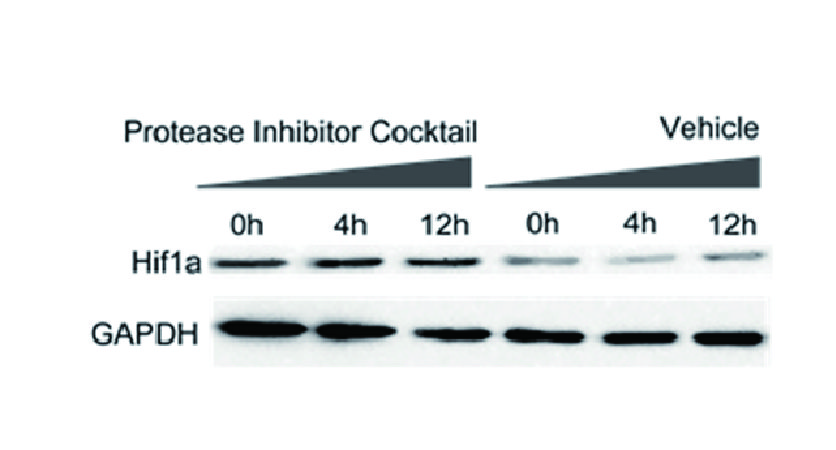
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Ivermectin B1a is the main component (not less than 80%) of the anthelmintic, ivermectin. Ivermectin is one of the most useful veterinary antiparasitic drugs ever produced. Ivermectin belongs to the macrocyclic lactone class of avermectins. Ivermectin contains two homologous compounds, H2B1a and H2B1b. Avermectins are potent insecticidal, anthelmintic and acaricidal compounds in mediating the paralysis of nematodes and certain classes of ectoparasites by increasing the membrane permeability to chlorine ions[1].
In humans, ivermectin has been used to treat African river blindness (onchocerciasis). Ivermectin significantly decreased the prevalence of skin and eye diseases linked to this infection [2]. Ivermectin B1a produced antiparasitic activity via an interaction with a common receptor molecule, glutamate-gated chloride channels, which virtually expressed on nematode neurons and pharyngeal muscle cells, inducing irreversible channel opening and very long-lasting hyperpolarization/depolarization of the neuron/muscle cell, thereby blocking further function. The EC50 of ivermectin was 104 nM [1,3].
References:
[1] J. Wolsetnholme and A. T. Rogers. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology131 Suppl, S85-S95 (2005).
[2] S. Gaisser, L. Kellenberger, A. L. Kaja, et al. Direct production of ivermectin-like drugs after domain exchange in the avermectin polyketide synthase of Streptomyces avermitilis ATCC31272. Organic & Biomolecular Chemistry 1(16), 2840-2847 (2003).
[3] J. P. Arena, K. K. Liu, P. S. Paress, et al. The mechanism of action of avermectins in Caenorhabditis elegans: Correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. Journal of Parasitology 81, 286-294 (1995).
| Physical Appearance | A white solid |
| Storage | Store at -20°C |
| M.Wt | 875.1 |
| Cas No. | 71827-03-7 |
| Formula | C48H74O14 |
| Synonyms | dihydro Avermectin B1a,22,23-dihydro Avermectin B1a |
| Solubility | Soluble in DMSO |
| Chemical Name | 5-O-demethyl-22,23-dihydro-avermectin A1a |
| SDF | Download SDF |
| Canonical SMILES | C/C([C@@H](O[C@@]1([H])C[C@H](OC)[C@@H](O[C@]2([H])O[C@@H](C)[C@H](O)[C@@H](OC)C2)[C@H](C)O1)[C@@H](C)/C=C/C=C(CO3)/[C@@]([C@@]3([H])[C@H](O)C(C)=C4)(O)[C@]4([H])C5=O)=C\C[C@]6([H])C[C@@](O5)([H])C[C@]7(CC[C@H](C)[C@]([C@H](CC)C)([H])O7)O6 |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
化学结构