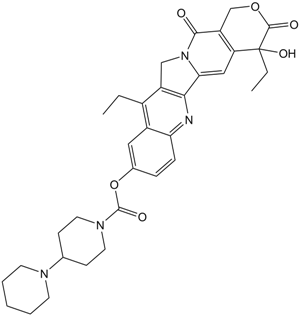
Irinotecan
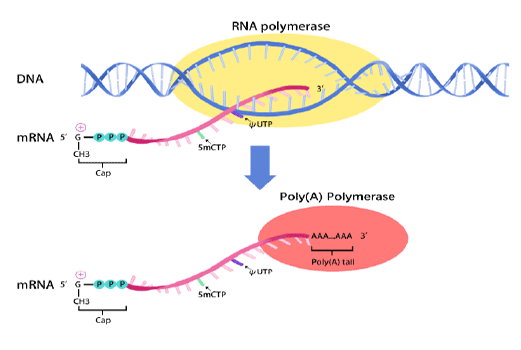
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
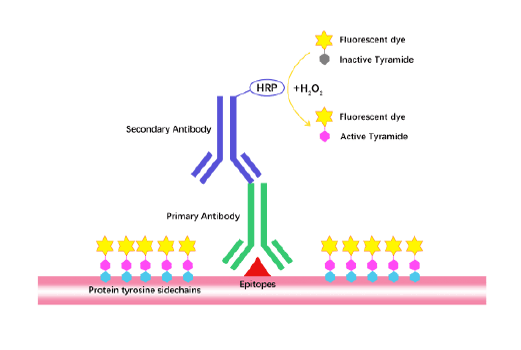
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
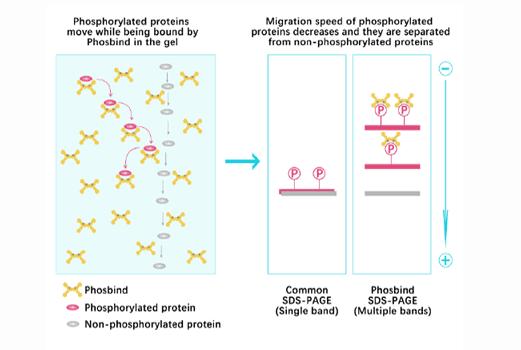
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
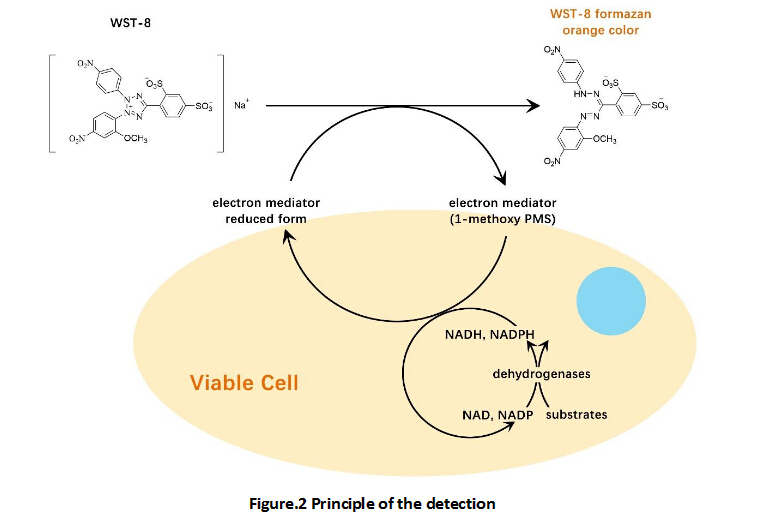
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
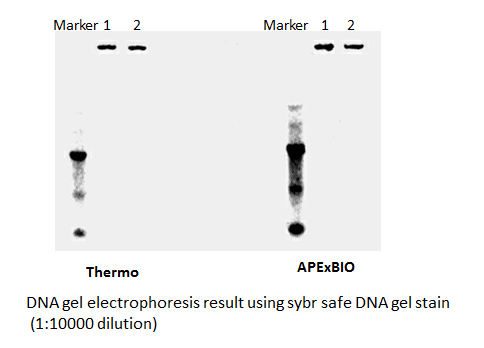
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
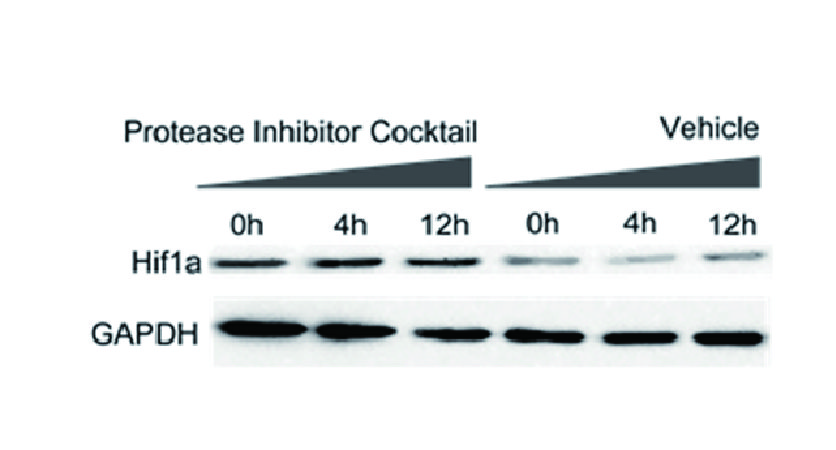
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Irinotecan(CPT-11)是一种用于治疗转移性结肠直肠癌的前体药物,是拓扑异构酶I的抑制剂,针对LoVo细胞和HT-29细胞的IC50分别为15.8 μM和5.17 μM [1]。在体内,Irinotecan通过羧酸酯酶转化酶(CCE)转化为其最具活性的代谢物SN-38 [2]。
体外实验:Irinotecan在LoVo细胞和HT-29细胞系中诱导相似量的切割复合物,IC50分别为15.8 μM和5.17 μM[1]。向血浆中加入157 mM Irinotecan后,在前60分钟期间,SN-38浓度显示线性增加,随后进入平台期。在前60分钟,转化率的平均值和标准偏差为515.9 ± 50.1 pmol/ml/h(n = 69),变异系数为0.097 [2]。相较于NSCLC细胞系,Irinotecan(CPT-11)对SCLC具有更加显著的活性(P = 0.0036)。在人肺癌细胞系中,CE活性似乎与对CPT-11的较高敏感性相关,并且可以部分地解释SCLC和NSCLC细胞之间对CPT-11的体外敏感性的差异[3]。在LS174T和COLO 320细胞中,CPT-11和SN-38的灵敏度最高,对SW1398细胞的灵敏度处于中间值,在COLO 205和WiDr细胞中最低。SN-38的活性是CPT-11的130-570倍[4]。
在体实验:在COLO 320异种移植物中,Irinotecan诱导92%的最大生长抑制[4]。单剂量的Irinotecan显著增加了在胃、十二指肠、结肠和肝脏中与DNA共价结合的拓扑异构酶I的量。同时,与对照组相比,在结肠粘膜细胞中,Irinotecan诱导显著更多的DNA链断裂[5]。
参考文献:
[1]. Tobin P, Clarke S, Seale J P, et al. The in vitro metabolism of irinotecan (CPT‐11) by carboxylesterase and β‐glucuronidase in human colorectal tumours[J]. British journal of clinical pharmacology, 2006, 62(1): 122-129.
[2]. Shingyoji M, Takiguchi Y, Watanabe‐Uruma R, et al. In vitro conversion of irinotecan to SN‐38 in human plasma[J]. Cancer science, 2004, 95(6): 537-540.
[3]. van Ark-Otte J, Kedde M A, Van Der Vijgh W J, et al. Determinants of CPT-11 and SN-38 activities in human lung cancer cells[J]. British journal of cancer, 1998, 77(12): 2171.
[4]. Jansen W J M, Zwart B, Hulscher S T M, et al. CPT-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants[J]. International journal of cancer, 1997, 70(3): 335-340.
[5]. Na Y S, Jung K A, Kim S M, et al. The histone deacetylase inhibitor PXD101 increases the efficacy of irinotecan in in vitro and in vivo colon cancer models[J]. Cancer chemotherapy and pharmacology, 2011, 68(2): 389-398.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 586.68 |
| Cas No. | 97682-44-5 |
| Formula | C33H38N4O6 |
| Solubility | insoluble in H2O; ≥11.4 mg/mL in DMSO; ≥4.9 mg/mL in EtOH |
| Chemical Name | 4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl [1,4'-bipiperidine]-1'-carboxylate |
| SDF | Download SDF |
| Canonical SMILES | O=C(OC1)C(O)(CC)C2=C1C(N(CC3=C4N=C5C(C=C(OC(N6CCC(N7CCCCC7)CC6)=O)C=C5)=C3CC)C4=C2)=O |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 细胞实验 [1]: | |
|
细胞系 |
HT29,NMG64/84,COLO-357,MIA PaCa-2,PANC-1细胞 |
|
溶解方法 |
该化合物在DMSO中的溶解度大于29.4 mg/mL。若获取更高浓度的溶液,可在37℃下孵育10分钟,随后在超声波浴中摇匀。-20℃以下可储存数月。 |
|
反应条件 |
0.1-1000 μg/ml,30 min |
|
应用 |
在所有测试的细胞系中,Irinotecan显示浓度和时间依赖性的细胞毒性作用。在COLO-357、MIA PaCa-2和PANC-1细胞中,Irinotecan增加G0/G1期的细胞数量,并减少S-和G2期细胞数。在HT29和NMG 64/84细胞中,低浓度的Irinotecan增加G2期细胞数。 |
| 动物实验 [2]: | |
|
动物模型 |
ICR雄性小鼠 |
|
给药剂量 |
腹腔注射,100 mg/kg |
|
应用 |
Irinotecan(100mg/kg,i.p.)注射后体重变化的时间过程表现出显著的给药时间依赖性差异(P <0.01)。在第3天和第4天之间注射Irinotecan(CPT-11)后,平均体重减轻最多。在1700小时后注射Irinotecan(CPT-11)后平均体重减轻程度最小。 |
|
注意事项 |
由于实验环境的不同,实际溶解度可能与理论值略有不同,请测试室内所有化合物的溶解度。 |
|
References: [1]. HOFMANN C, BUTTENSCHOEN K, STRAETER J, et al. Pre-clinical evaluation of the activity of irinotecan as a basis for regional chemotherapy[J]. Anticancer research, 2005, 25(2A): 795-804. [2]. Ohdo S, Makinosumi T, Ishizaki T, et al. Cell cycle-dependent chronotoxicity of irinotecan hydrochloride in mice[J]. Journal of Pharmacology and Experimental Therapeutics, 1997, 283(3): 1383-1388. |
|
质量控制和MSDS
- 批次:
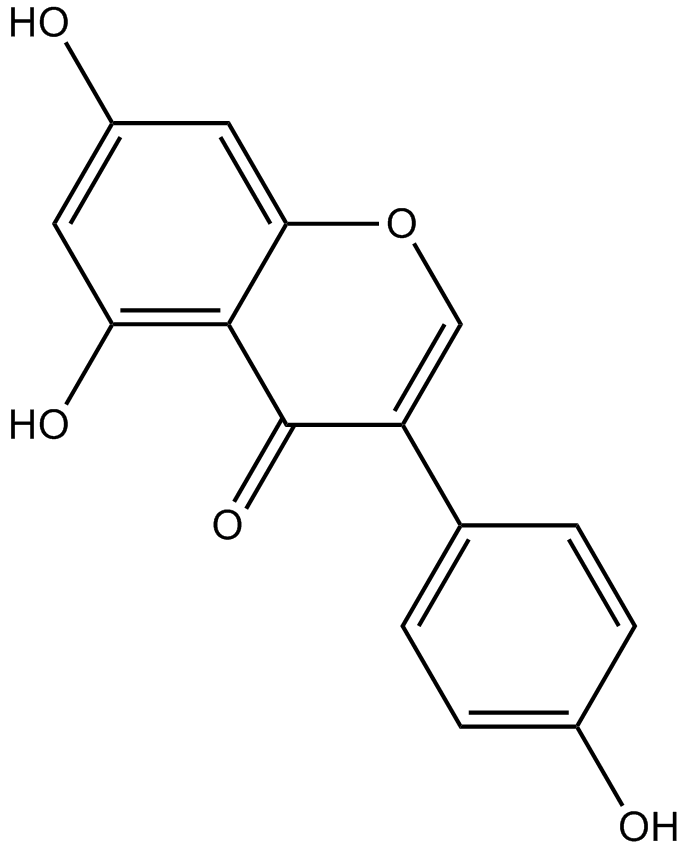
化学结构