GSK429286A
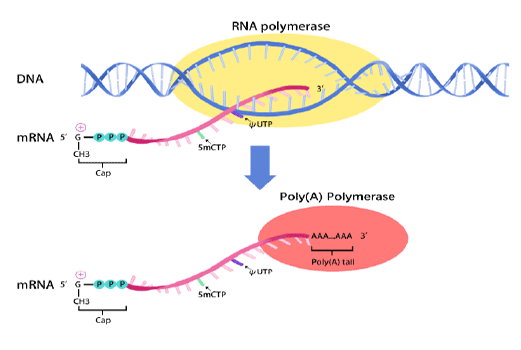
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
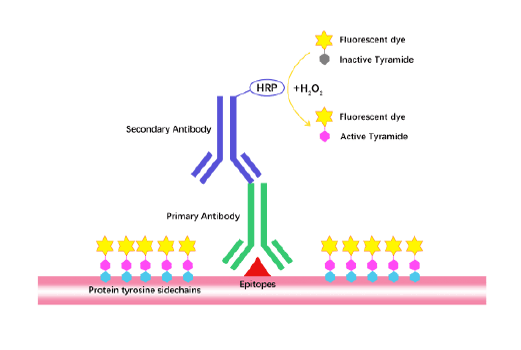
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
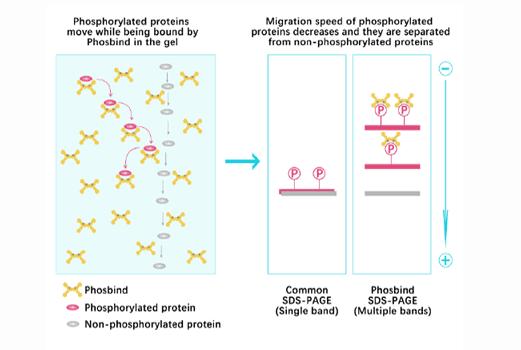
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
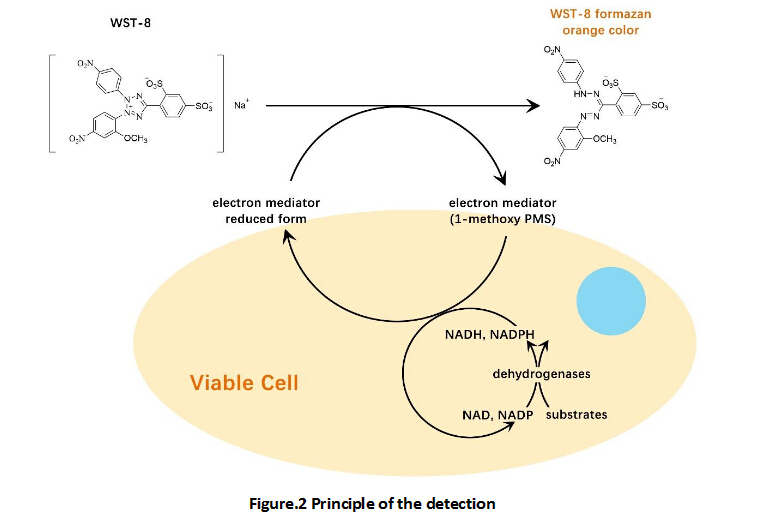
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
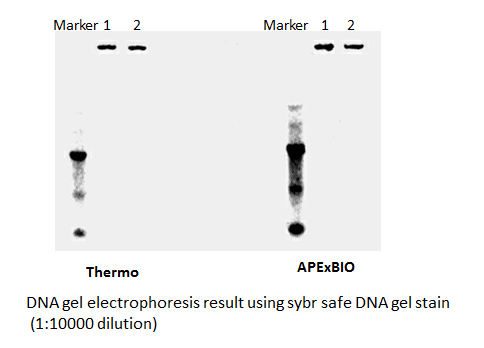
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
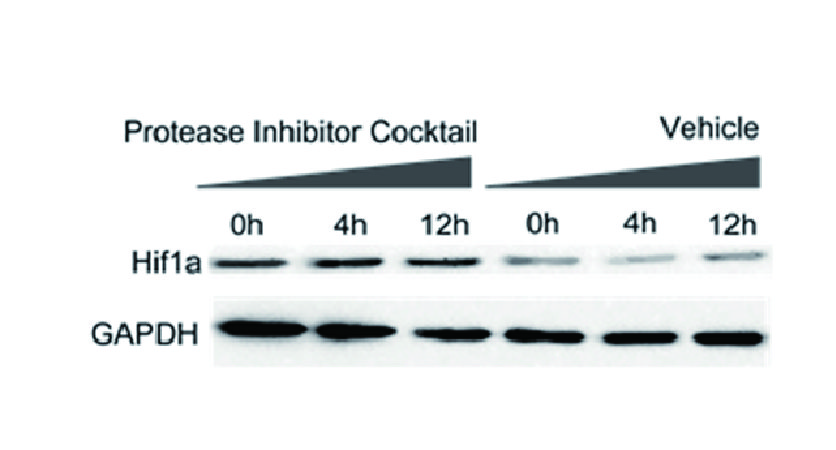
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
GSK429286A是一种选择性ROCK1和ROCK2抑制剂,IC50值分别为14 nM和63 nM [1]。
Rho-激酶(ROCK) 属于AGC家族(蛋白激酶A、蛋白激酶G和蛋白激酶C)的成员,在促进肌动蛋白肌球蛋白介导的收缩力产生中具有重要作用[2]。
GSK429286A是一种有效ROCK抑制剂,与已报道的ROCK抑制剂Y27632活力不同。GST实验表明,10 μM GSK429286A通过抑制ROCK,可增加MYPT在Thr850位点的磷酸化作用[3]。
自发性高血压雄性Sprague-Dawley大鼠模型实验中,口服给药GSK429286A (30 mg/kg)可降低平均动脉压,治疗2小时左右,最大降压可达到50 mmHg[4]。
参考文献:
[1] Nichols, R. J., et al., Substrate specificity and inhibitors of LRRK2, a protein kinase mutated in Parkinson's disease. Biochem J, 2009. 424(1): p. 47-60.
[2] Shi, J. , et al., Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis, 2013. 4: p. e483.
[3] Davis, D. A., et al., Increased therapeutic potential of an experimental anti-mitotic inhibitor SB715992 by genistein in PC-3 human prostate cancer cell line. BMC Cancer, 2006. 6: p. 22.
[4] Goodman, K. B., et al., Development of dihydropyridone indazole amides as selective Rho-kinase inhibitors. J Med Chem, 2007. 50(1): p. 6-9.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 432.37 |
| Cas No. | 864082-47-3 |
| Formula | C21H16F4N4O2 |
| Solubility | ≥21.6 mg/mL in DMSO; insoluble in H2O; ≥2.73 mg/mL in EtOH with gentle warming and ultrasonic |
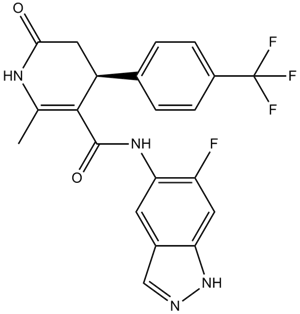
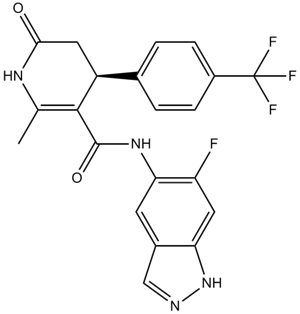
| Chemical Name | N-(6-fluoro-1H-indazol-5-yl)-6-methyl-2-oxo-4-[4-(trifluoromethyl)phenyl]-3,4-dihydro-1H-pyridine-5-carboxamide |
| SDF | Download SDF |
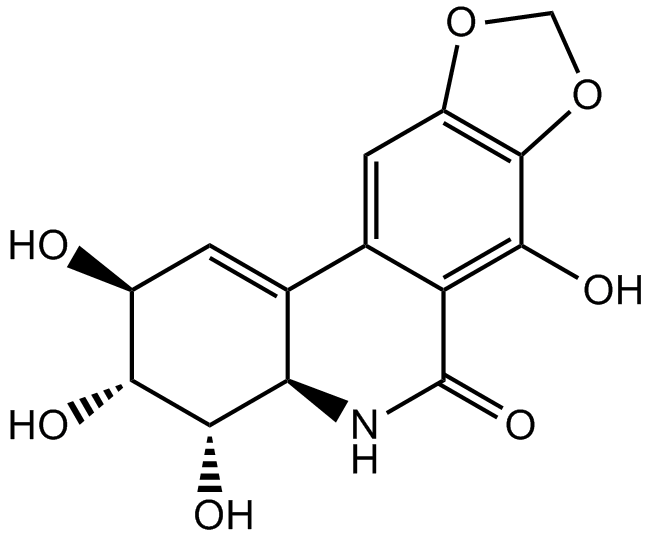
| Canonical SMILES | CC1=C(C(CC(=O)N1)C2=CC=C(C=C2)C(F)(F)F)C(=O)NC3=C(C=C4C(=C3)C=NN4)F |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 细胞实验[1]: | |
|
细胞系 |
MDA-MB-231 (TRPM7 shRNA) 细胞和MCF7 (TRPM7 shRNA)细胞 |
|
制备方法 |
该化合物在DMSO中的溶解度大于10 mM,若配制更高浓度的溶液,一般步骤如下:请将试管置于37℃加热10分钟和/或将其置于超声波浴中震荡一段时间。原液于-20℃可放置数月。 |
|
反应条件 |
1 μM,24 hours |
|
实验结果 |
GSK429286A通过抑制Rho激酶,恢复血清诱导的TRPM7敲低细胞的transwell迁移,而不影响MDA-MB-231对照细胞的迁移。同样,抑制Rho激酶拯救了MFC7 TRPM7 shRNA细胞的间隙闭合速度。与MDA-MB-231细胞相比,低浓度的GSK429286A显著增加MCF7对照细胞的间隙闭合速度。 |
| 动物实验[2]: | |
|
动物模型 |
雄性Sprague-Dawley大鼠 |
|
给药剂量 |
口服,30 mg/kg |
|
实验结果 |
GSK429286A具有61%的口服生物利用度,口服给药后,自发性高血压大鼠的平均动脉压显著降低。30 mg/kg口服给药后约2小时,平均动脉压最大减少50 mmHg。 |
|
注意事项 |
请于室内测试所有化合物的溶解度。虽然化合物的实际溶解度可能与其理论值略有不同,但仍处于实验系统误差的允许范围内。 |
|
References: [1] Middelbeek J, Kuipers A J, Henneman L, et al. TRPM7 is required for breast tumor cell metastasis. Cancer research, 2012, 72(16): 4250-4261. [2] Goodman K B, Cui H, Dowdell S E, et al. Development of dihydropyridone indazole amides as selective Rho-kinase inhibitors. Journal of medicinal chemistry, 2007, 50(1): 6-9. | |
| Description | GSK429286A是一种选择性ROCK1和ROCK2抑制剂,IC50值分别为14 nM和63 nM。 | |||||
| 靶点 | ROCK1 | ROCK2 | ||||
| IC50 | 14 nM | 63 nM | ||||
质量控制和MSDS
- 批次:
化学结构