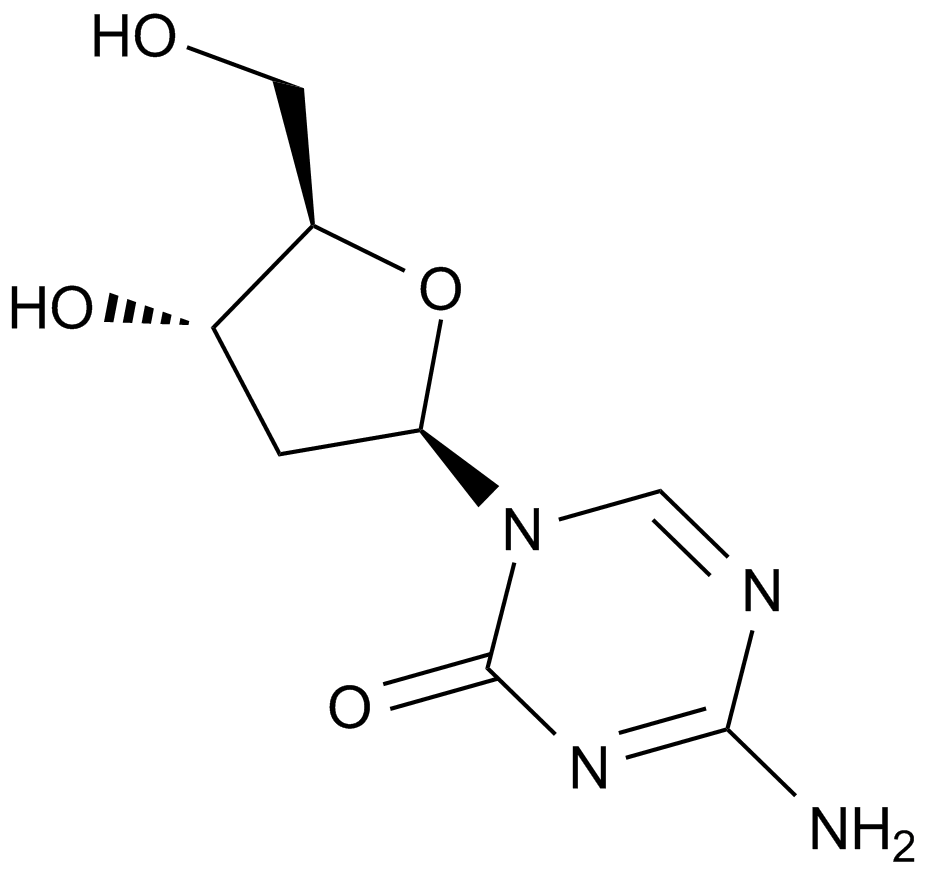
Decitabine (NSC127716, 5AZA-CdR)
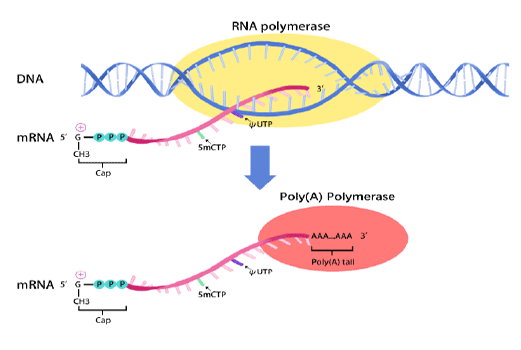
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
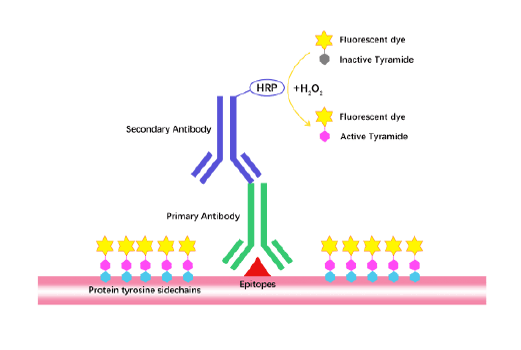
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
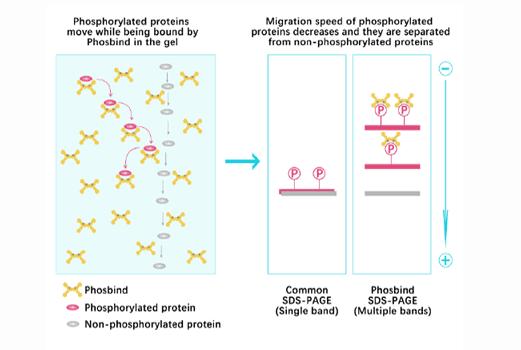
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
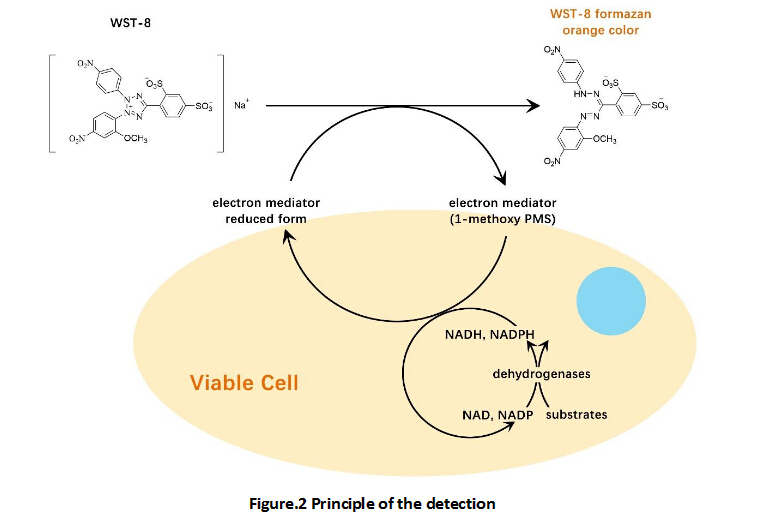
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
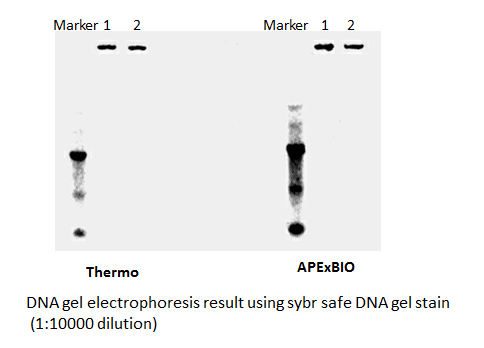
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
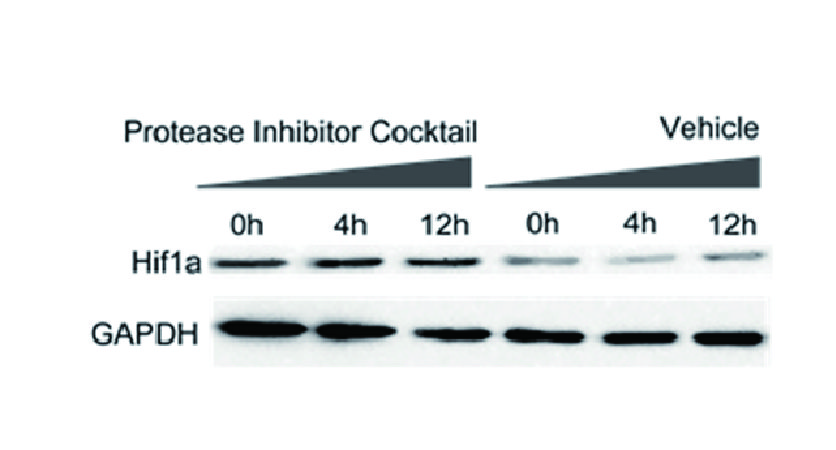
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Decitabine是一种脱氧胞苷类似物和细胞分化诱导剂。Decitabine通过不依赖于DNA甲基化的转录后机制增加γ-球蛋白的表达,从而能够掺入到DNA中,在靶向DNA甲基化的胞嘧啶位点与DNA甲基转移酶形成不可逆的共价键。据报道,decitabine在重新激活表观遗传学上沉默的肿瘤抑制基因方面具有实质性功效。在结肠癌细胞系HCT116和RKO中,decitabine分别增加未甲基化的hMLH1和MGMT启动子上组蛋白H3赖氨酸9乙酰化:甲基化的比率。在T24膀胱癌细胞中,decitabine可以增加未甲基化的p14启动子上组蛋白H3赖氨酸9的乙酰化和组蛋白H3赖氨酸4的甲基化。
参考文献:
Carlo Stresemann, Frank Lyko. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. International Journal of Cancer. 2008; 123(1): 8 – 13.
Jean-Pierre J. Issa, Guillermo Garcia-Manero, Francis J. Giles, Rajan Mannari, Deborah Thomas, Stefan Faderl, Emel Bayar, John Lyons, Craig S. Rosenfeld, Jorge Cortes, and Hagop M. Kantarjian. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2’-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004; 103 (5): 1635 – 40.
Hagop Kantarjian, Yasuhiro Oki, Guillermo Garcia-Manero, Xuelin Huang, Susan O’Brien, Jorge Cortes, Stefan Faderl, Carlos Bueso-Ramos, Farhad Ravandi, Zeev Estrov, Alessandra Ferrajoli, William Wierda, Jianqin Shan, Jan Davis,Francis Giles, Hussain I. Saba, and Jean-Pierre J. Issa. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007; 109 (1): 52 – 57.
Stuart A. Scotta, Wei-Feng Donga, Calley Hirscha, David Sheridana, Stephen E. Sanchea, C. Ronald Geyera, John F. DeCoteau. 5-Aza-2-deoxycytidine (decitabine) can relieve p21WAF1 repression in human acute myeloid leukemia by a mechanism involving release of histone deacetylase 1 (HDAC1) without requiring p21WAF1 promoter demethylation. Leukemia Research. 2006; 30(1): 69 – 76.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 228.08 |
| Cas No. | 2353-33-5 |
| Formula | C8H12N4O4 |
| Synonyms | 5-Aza-2'-deoxycytidine;Decitabine |
| Solubility | insoluble in EtOH; ≥11.4 mg/mL in DMSO; ≥23.3 mg/mL in H2O with gentle warming |
| Chemical Name | 4-amino-1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one |
| SDF | Download SDF |
| Canonical SMILES | C1C(C(OC1N2C=NC(=NC2=O)N)CO)O |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 细胞实验[1]: | |
|
细胞系 |
人类和小鼠黑色素瘤细胞(A375 and B16). |
|
溶解方法 |
该化合物在DMSO中的溶解度大于10 mM。若配制更高浓度的溶液,一般步骤如下:请将试管置于37℃加热10分钟和/或将其置于超声波浴中震荡一段时间。原液于-20℃可放置数月。 |
|
反应条件 |
在第1天和第4天加入0.5 μM的decitabine,在第8天获得图像。 |
|
实验结果 |
Decitabine减少黑素瘤细胞系的细胞增殖并诱导分化的形态学变化。 |
| 动物实验[2]: | |
|
动物模型 |
U2OS肿瘤异种移植小鼠 |
|
剂量 |
在第29、31和33天腹膜内给药2.5 mg/kg。在第37天,处死小鼠。 |
|
溶解方法 |
溶于生理盐水(0.9% w/v NaCl) |
|
实验结果 |
Decitabine显著降低肿瘤异种移植物大小并降低有丝分裂活性,增加凋亡细胞数量和骨基质的生成量。Decitabine还增加促凋亡基因GADD45A、HSPA9B、PAWR、PDCD5、NFKBIA和TNFAIP3的表达(≥2倍)[2]。 |
|
注意事项 |
请测试所有化合物在室内的溶解度,实际溶解度和理论值可能略有不同。这是由实验系统的误差引起的,属于正常现象。 |
|
References: [1]. Alcazar O, Achberger S, Aldrich W, et al. Epigenetic regulation by decitabine of melanoma differentiation in vitro and in vivo. Int J Cancer, 2012, 131(1): 18-29. [2]. Al-Romaih K, Somers GR, Bayani J, et al. Modulation by decitabine of gene expression and growth of osteosarcoma U2OS cells in vitro and in xenografts: identification of apoptotic genes as targets for demethylation. Cancer Cell Int, 2007, 7: 14. |
|
质量控制和MSDS
- 批次:
化学结构