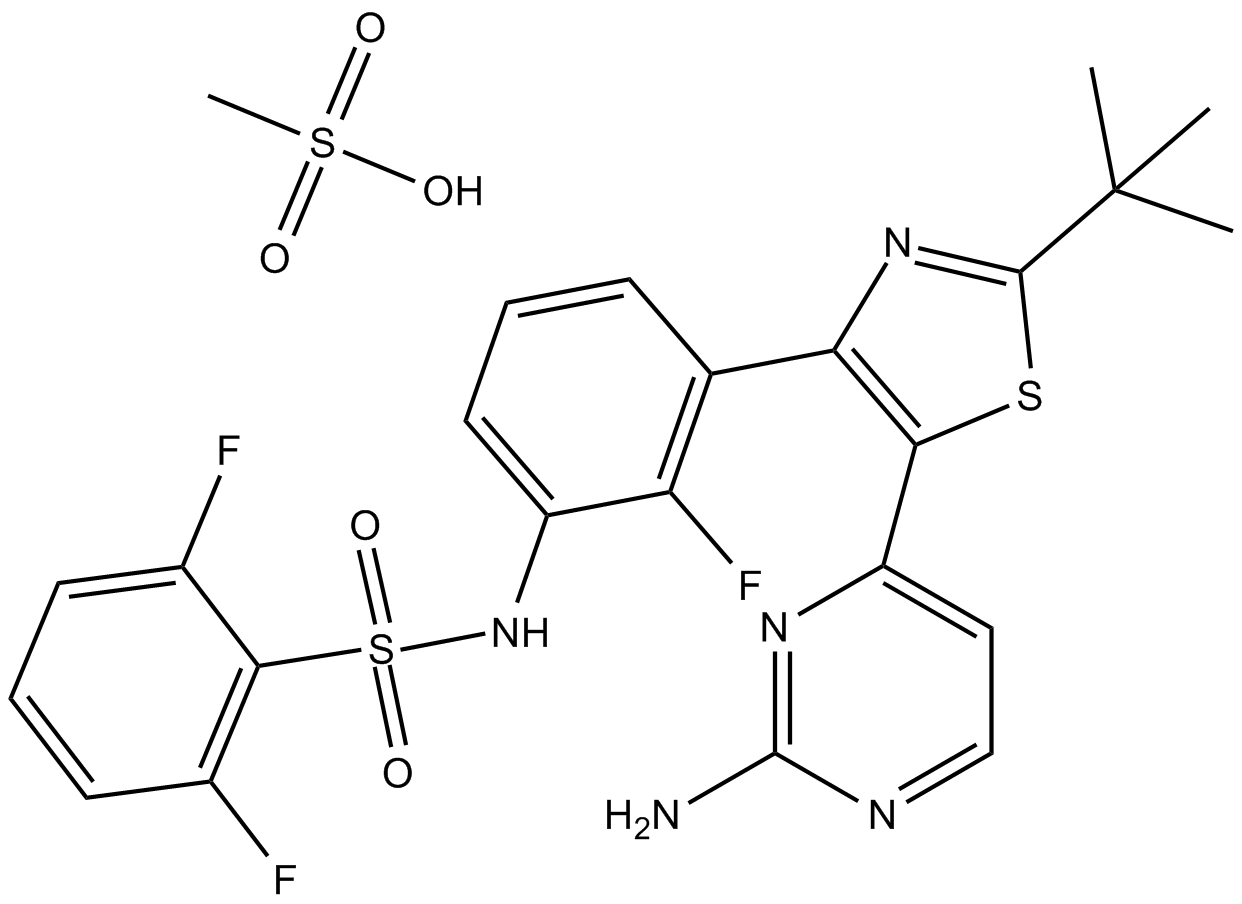
Dabrafenib Mesylate (GSK-2118436)
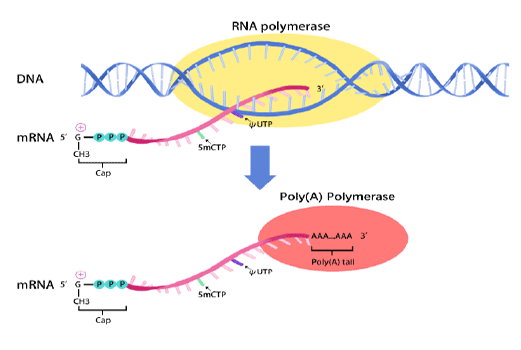
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
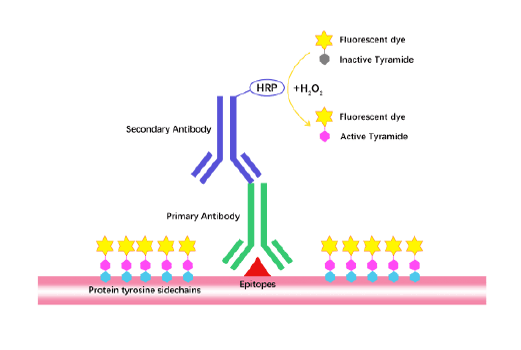
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
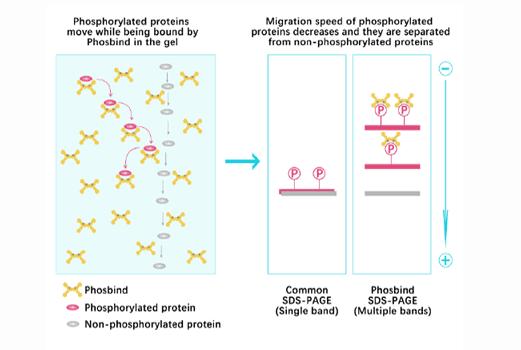
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
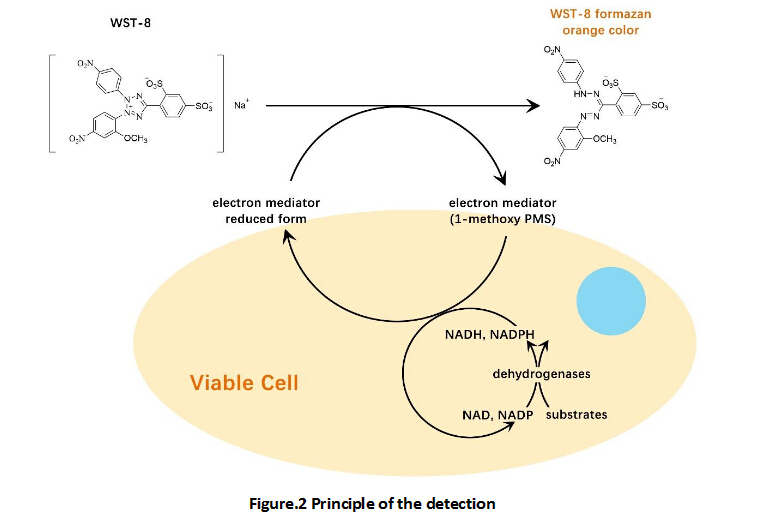
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
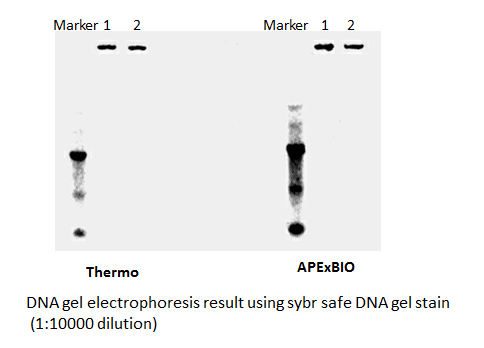
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
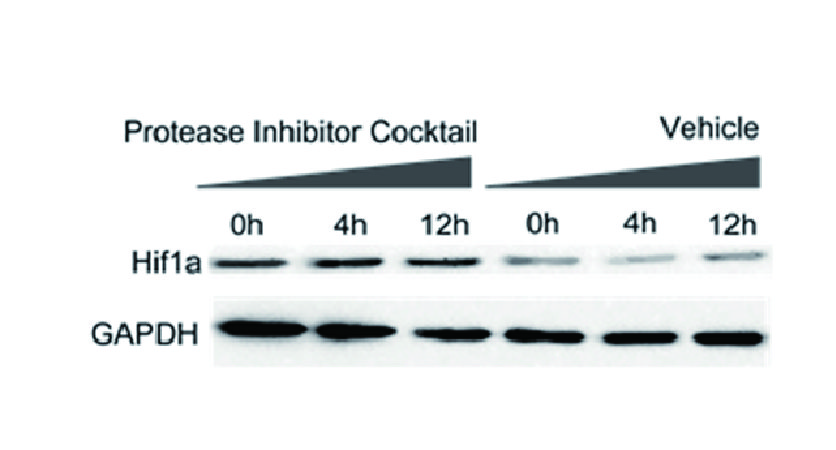
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
GSK2118436是一种选择性的和可口服的BRAF V600E抑制剂。与对野生型B-Raf和c-Raf(IC50值为3.2 nM和5.0 nM)的作用相比,GSK2118436对B-Raf V600E具有高选择性,IC50值为0.8 nM[4]。BRAF是一种丝氨酸/苏氨酸蛋白激酶,在调控MAPK/ERK信号通路中起重要作用。BRAF突变在许多人类癌症中频繁发生[1, 2]。BRAF V600E突变具有持续活性,使得MAPK/ERK的激活不依赖于上游信号[3]。
GSK2118436结合并抑制Raf家族激酶的活性。GSK2118436选择性地抑制MAPK/ERK激活,抑制细胞增殖、转化和致瘤性。FDA于2013年5月30日已批准GSK2118436作为单药用于治疗BRAF V600E突变的晚期黑色素瘤。
参考文献:
[1]Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, Ohtsuru A, Saenko VA, Kanematsu T, Yamashita S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. 2003; 88 (9): 4393–7.
[2]Tan YH, Liu Y, Eu KW, Ang PW, Li WQ, Salto-Tellez M, Iacopetta B, Soong R. Detection of BRAF V600E mutation by pyrosequencing. Pathology 2008; 40 (3): 295–8.
[3]Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417: 949-954.
[4]Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, Lazova R, Klump V, Pawelek JM, Xu X, Xu W, Schuchter LM, Davies MA, Herlyn M, Winkler J, Koumenis C, Amaravadi RK. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest. 2014; 124(3): 1406-17.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 615.67 |
| Cas No. | 1195768-06-9 |
| Formula | C24H24F3N5O5S3 |
| Synonyms | GSK-2118436 Mesylate;GSK2118436 Mesylate;GSK 2118436 Mesylate;GSK 2118436B,Tafinlar, |
| Solubility | ≥30.75 mg/mL in DMSO; insoluble in H2O; ≥2.74 mg/mL in EtOH with gentle warming and ultrasonic |
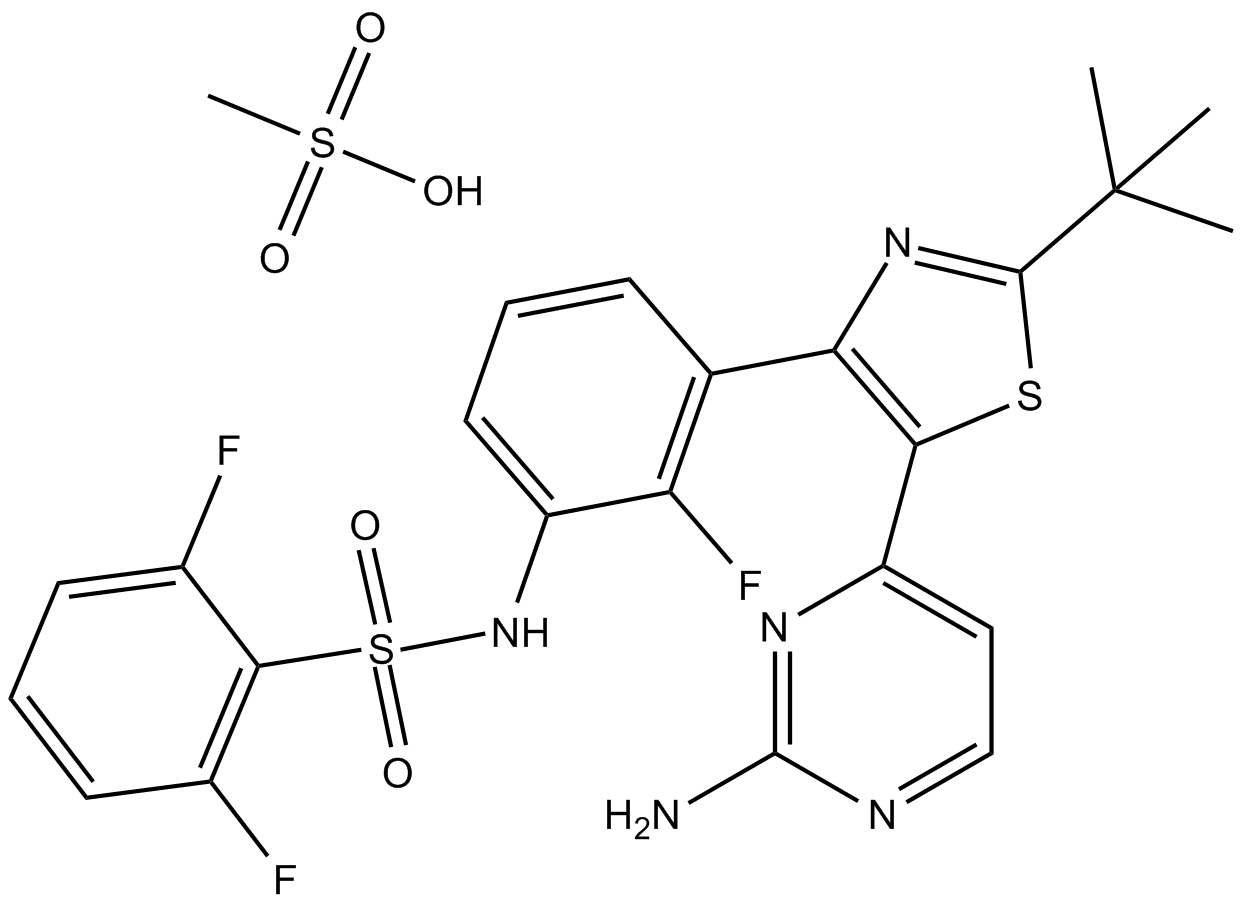
| Chemical Name | N-[3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide;methanesulfonic acid |
| SDF | Download SDF |
| Canonical SMILES | CC(C)(C)C1=NC(=C(S1)C2=NC(=NC=C2)N)C3=C(C(=CC=C3)NS(=O)(=O)C4=C(C=CC=C4F)F)F.CS(=O)(=O)O |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 细胞实验[1]: | |
|
细胞系 |
M257野生型BRAF、LCP BRAF V600E和WM266 BRAF V600D黑素瘤细胞系。 |
|
溶解方法 |
可溶于DMSO。若配制更高浓度的溶液,一般步骤如下:请将试管置于37℃加热10分钟和/或将其置于超声波浴中震荡一段时间。原液于-20℃可放置数月 |
|
反应条件 |
3-100 nM;72 h |
|
实验结果 |
在携带突变BRAF的黑色素瘤细胞系中,Dabrafenib显著抑制细胞增殖和磷酸化ERK。 |
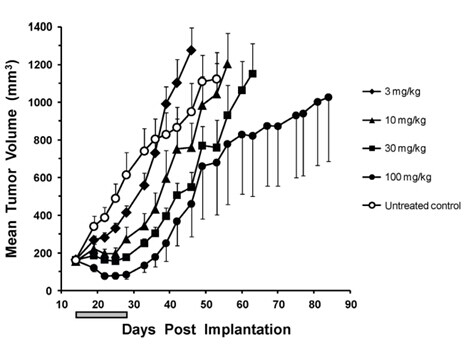
| 动物实验 [2]: | |
|
动物模型 |
异种移植BRAF V600E(A375P)人肿瘤的雌性CD1 nu/nu小鼠。 |
|
剂量 |
30 mg/kg;14 days;每天一次;口服 |
|
溶解方法 |
0.5%羟丙基甲基纤维素,0.2% Tween 80,溶于pH 8.0蒸馏水,每20g体重给药0.2 mL。 |
|
实验结果 |
Dabrafenib是一种口服生物可利用药物,降低pERK并抑制肿瘤生长。Dabrafenib使pERK和Ki67分别降低89%和28%,并将p27增加54%。 |
|
注意事项 |
请测试室内所有化合物的溶解度,实际溶解度可能与理论值略有不同。这是由实验系统错误引起的,这是正常的。 |
|
References: [1]. Gentilcore G, Madonna G, Mozzillo N, et al. Effect of dabrafenib on melanoma cell lines harbouring the BRAF(V600D/R) mutations. BMC Cancer, 2013, 13: 17. [2]. King AJ, Arnone MR, Bleam MR, et al. Dabrafenib; preclinical characterization, increased efficacy when combined with trametinib, while BRAF/MEK tool combination reduced skin lesions. PLoS One, 2013, 8(7): e67583. |
|
| Targets | Raf | |||||
| IC50 | 3.2/0.8/5.0 nM (B-Raf/B-RafV600E/ c-Raf) |
质量控制和MSDS
- 批次:
化学结构
相关生物数据