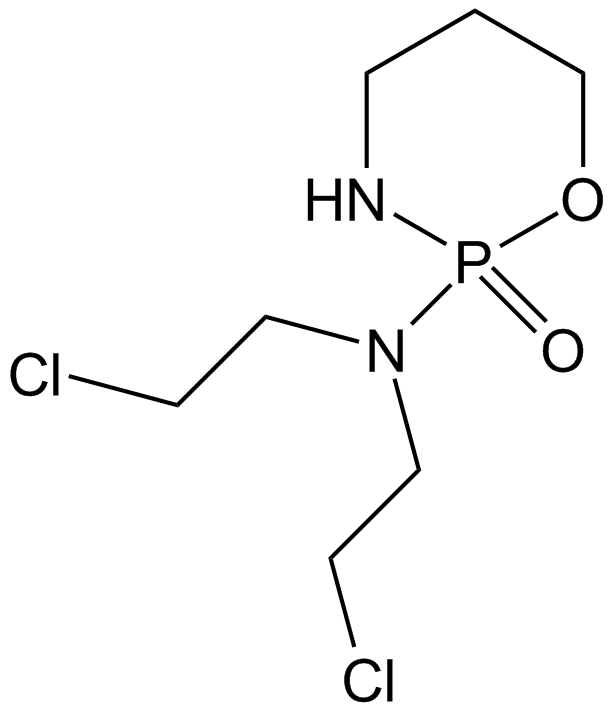
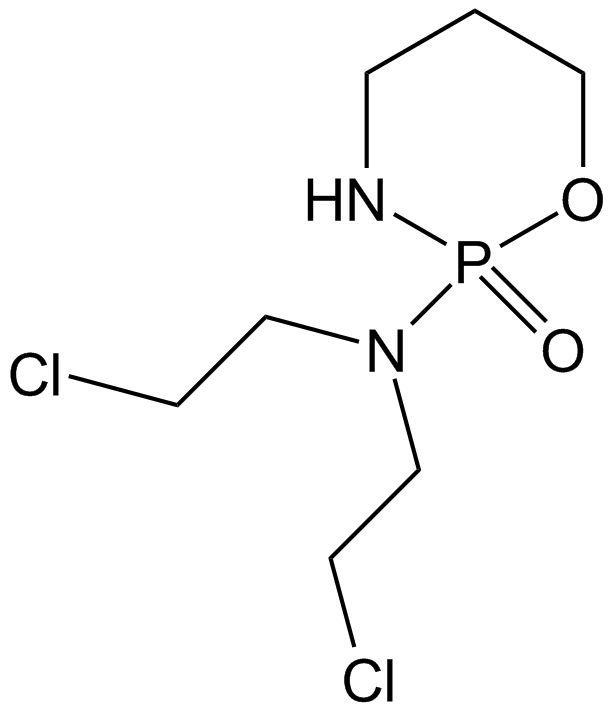
Cyclophosphamide
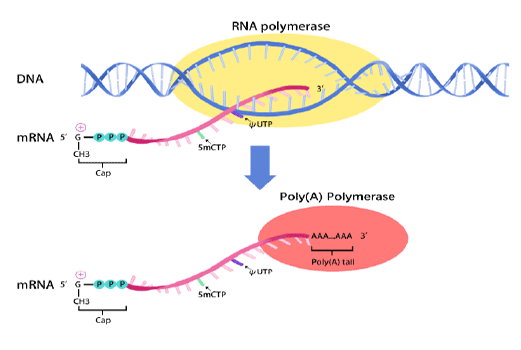
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
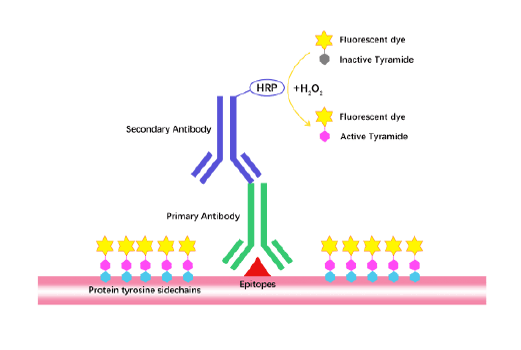
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
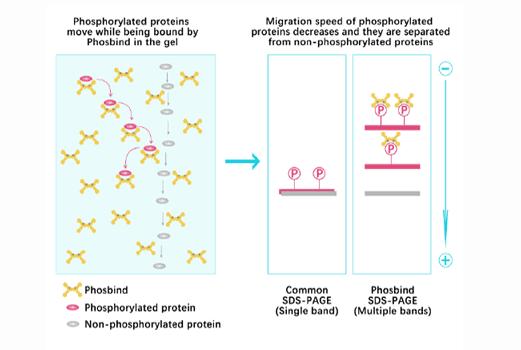
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
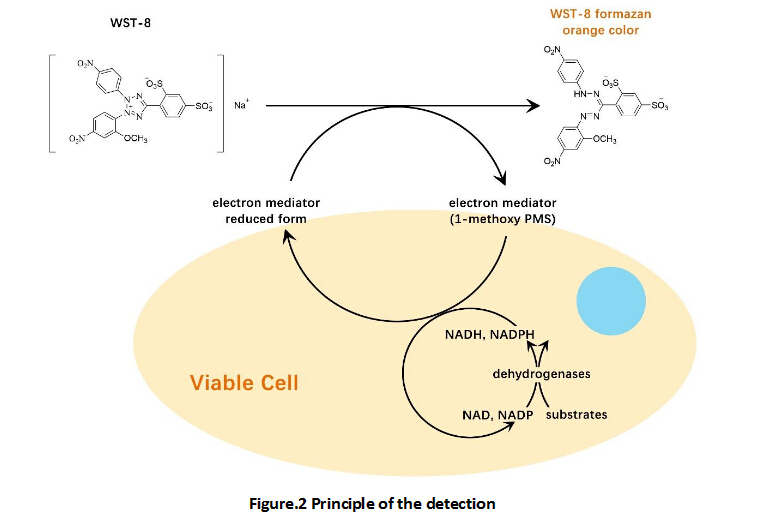
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
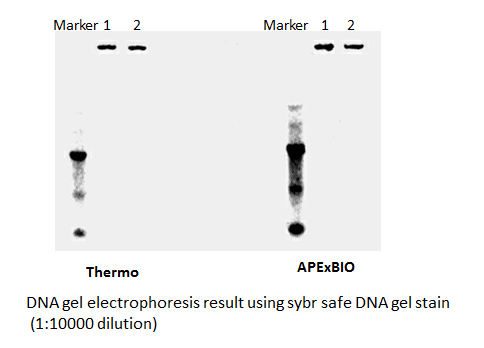
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
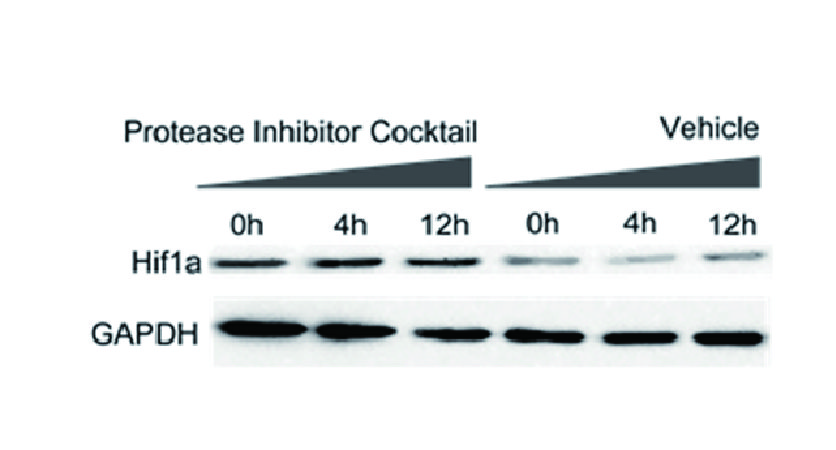
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Cyclophosphamide, an inactive prodrug, is a kind of nitrogen mustard alkylating agent. Cyclophosphamide requires enzymatic and chemical activation. As a result, nitrogen mustard is produced. It causes DNA cross-linking that accounts for its cytotoxic properties. [1] IC50 of cytotoxicity in mouse embryo BALB/c 3T3 cells is 37.6 μM, [2] IC50 of cytotoxicity against human HL60 cells is 8.79 μM measured by MTT assay. [3]
Cyclophosphamide attaches the alkyl group to the guanine base of DNA causing its crosslinking, strand breakage and inducing mutations.
In vitro, cyclophosphamide has a dose-dependent, bimodal effect on the immune system. Low-dose cyclophosphamide not only decreases cell number but leads to decreased functionality of regulatory T cells (TREGs). Cyclophosphamide treatment enhances apoptosis and decreases homeostatic proliferation of these cells. Expression of GITR and FoxP3, which are involved in the suppressive activity of TREGs, is down-regulated after cyclophosphamide administration.[4] In primary human hepatocyte cultures, cyclophosphamide increases CYP3A4, CYP2C8, and CYP2C9 protein levels, causing its 4-hydroxylation rate enhance.[5] In somatic cells, cyclophosphamide produces gene mutations, chromosome aberrations, micronuclei and sister chromatid exchanges in a variety of cultured cells in the presence of metabolic activation as well as sister chromatid exchanges without metabolic activation. [6]
In vivo, it has produced chromosome damage and micronuclei in rats, mice and Chinese hamsters, and gene mutations in the mouse spot test and in the transgenic lacZ construct of Muta™Mouse. [6]
References:
[1] Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol. 2009 Nov; 6 (11):638-47.
[2] Moon KY, Kwon CH. N3-methyl-mafosfamide as a chemically stable, alternative prodrug of mafosfamide. Bioorg Med Chem Lett. 1998 Jul 7; 8 (13):1673-8.
[3] Patel MM, Mali MD, Patel SK. Bernthsen synthesis, antimicrobial activities and cytotoxicity of acridine derivatives. Bioorg Med Chem Lett. 2010 Nov 1; 20 (21):6324-6.
[4] Lutsiak ME, Semnani RT, De Pascalis R,et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005 Apr 1; 105 (7):2862-8. Epub 2004 Dec 9.
[5] Chang TK, Yu L, Maurel P, Waxman DJ. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997 May 15; 57 (10):1946-54.
[6] Anderson D, Bishop JB, Garner RC, et al. Cyclophosphamide: review of its mutagenicity for an assessment of potential germ cell risks. Mutat Res. 1995 Aug; 330 (1-2):115-81.
| Physical Appearance | A solid |
| Storage | Store at -20°C, 该产品在溶液中不稳定,建议现配现用 |
| M.Wt | 261.09 |
| Cas No. | 50-18-0 |
| Formula | C7H15Cl2N2O2P |
| Solubility | ≥11.85 mg/mL in H2O with gentle warming and ultrasonic; ≥13.05 mg/mL in DMSO; ≥50.8 mg/mL in EtOH |
| Chemical Name | N,N-bis(2-chloroethyl)-2-oxo-1,3,2λ5-oxazaphosphinan-2-amine |
| SDF | Download SDF |
| Canonical SMILES | C1CNP(=O)(OC1)N(CCCl)CCCl |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| Cell experiment:[1] | |
|
Cell lines |
9L gliosarcoma cells retrovirally transduced with CYP2B6 |
|
Reaction Conditions |
1 mM cyclophosphamide for 48 h incubation |
|
Applications |
Cyclophosphamide was shown to cause tumor cell death by stimulating apoptosis, as evidenced by the induction of plasma membrane blebbing, DNA fragmentation, and cleavage of the caspase 3 and caspase 7 substrate poly(ADP-ribose) polymerase in drug-treated cells. |
| Animal experiment:[2] | |
|
Animal models |
Female C57BL/6 mice, 8 weeks of age |
|
Dosage form |
2 mg Injected intraperitoneally |
|
Applications |
Low-dose cyclophosphamide not only decreased the number of regulatory T cells (TREGs), but also led to decreased functionality of TREGs. Cyclophosphamide treatment enhanced apoptosis and decreased homeostatic proliferation of these cells. |
|
Note |
The technical data provided above is for reference only. |
|
References: 1. Schwartz PS, Waxman DJ. Cyclophosphamide induces caspase 9-dependent apoptosis in 9L tumor cells. Molecular Pharmacology, 2001, 60(6): 1268-1279. 2. Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood, 2005, 105(7): 2862-2868. |
|
质量控制和MSDS
- 批次:
化学结构