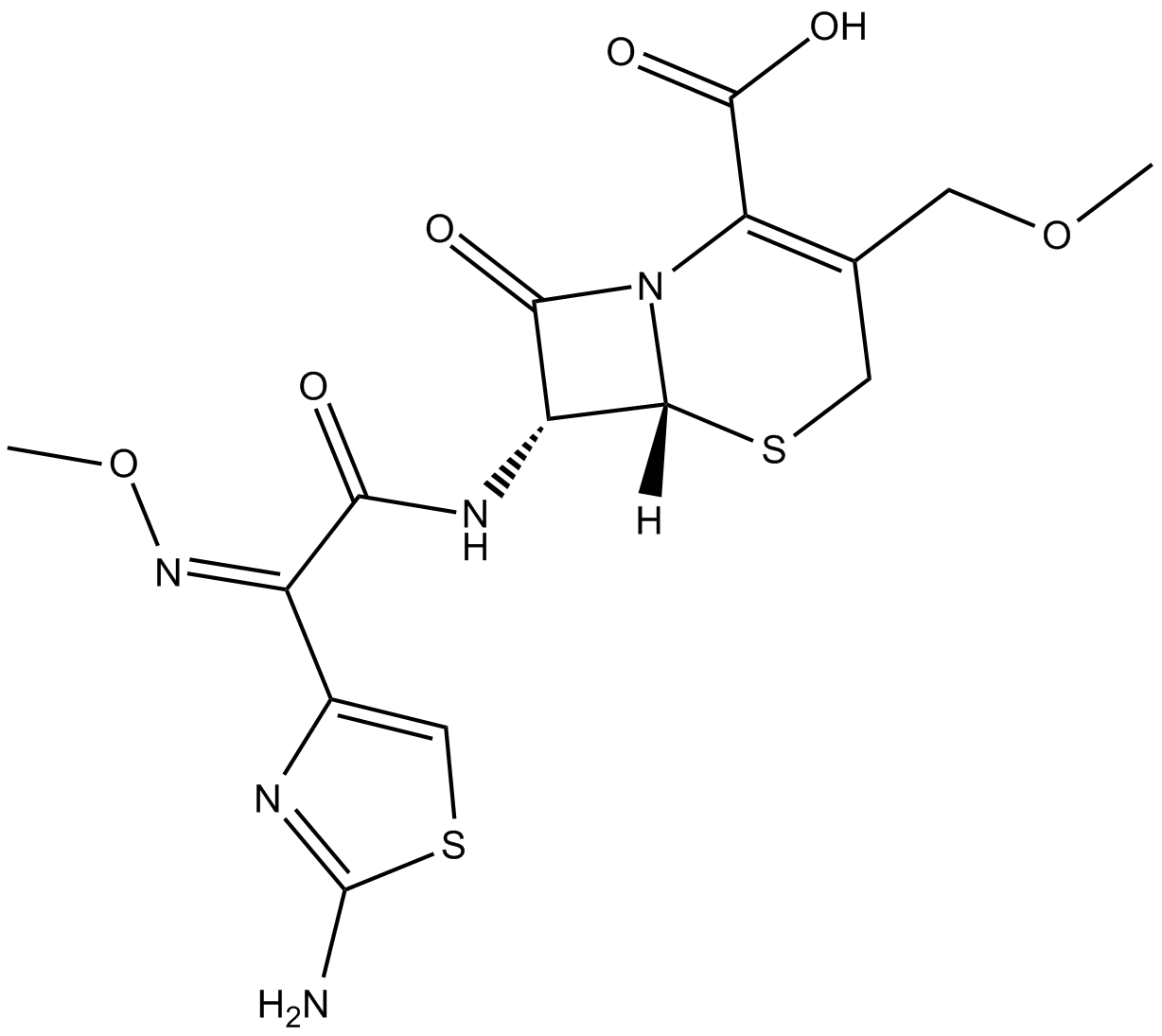
Cefpodoxime (free acid)
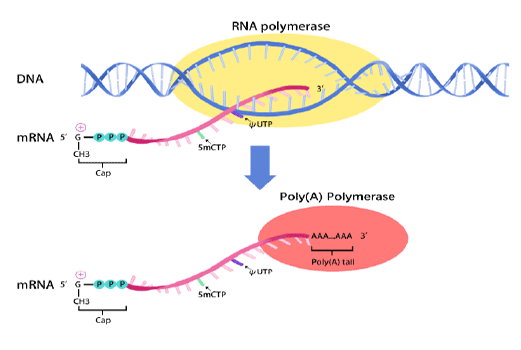
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
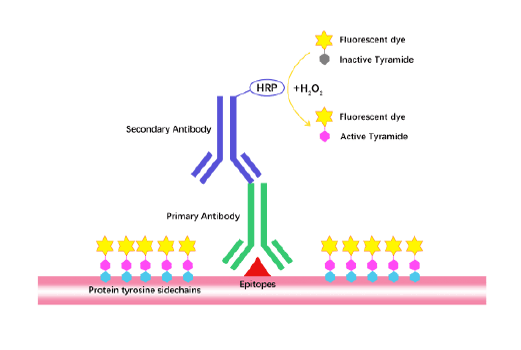
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
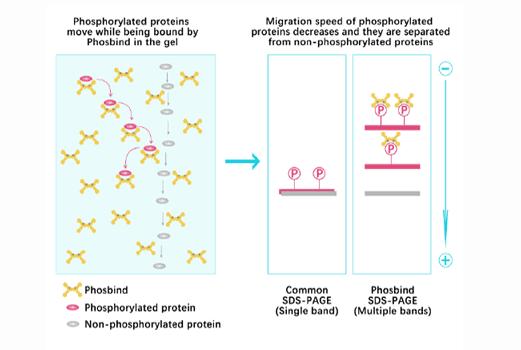
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
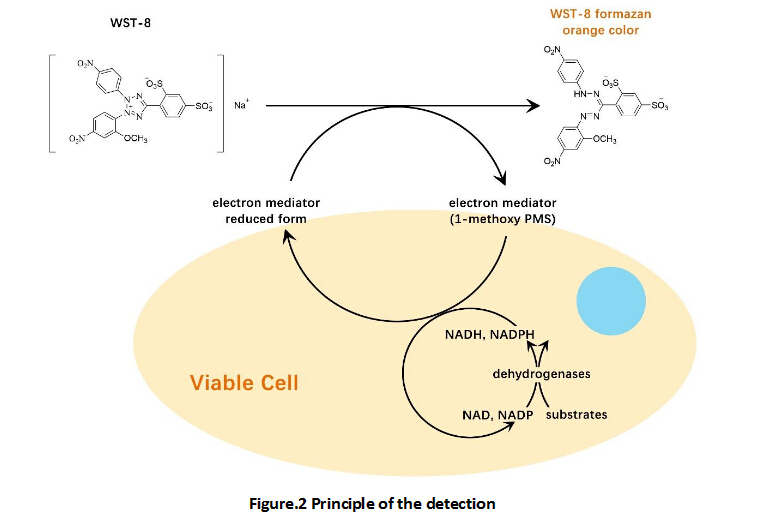
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
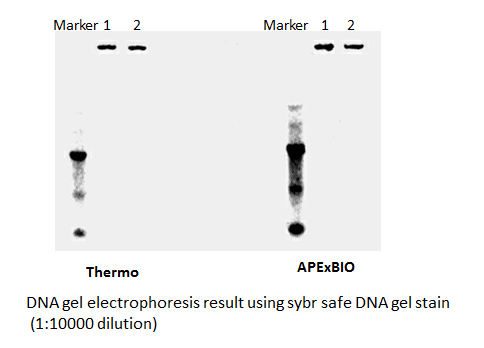
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
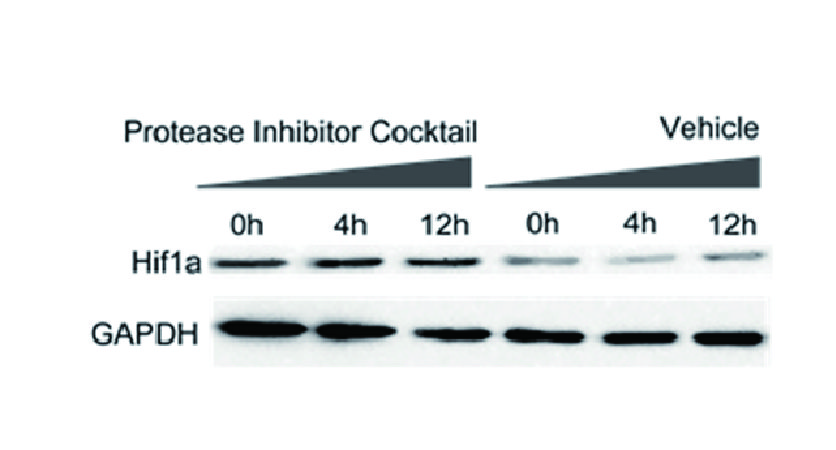
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Cefpodoxime, as known as R 3763, is a metabolite of cefpodoxime proxetil. It is demonstrated that cefpodoxime, as an oral third generation cephalosporin antibiotic, is active against most Gram-positive and Gram-negative bacteria.
Cefpodoxime suppresses bacterial septum and cell wall synthesis by binding to penicillin-binding proteins (PBPs) located in the bacterial cytoplasmic membrane.
In vitro: Cefpodoxime showed antibacterial activities against obligatory anaerobes and salmonella spp., shigella spp. and Neisseria meningitides. The activity of cefpodoxime was less active than R95867, an active form of CS-834, against Gram-negative bacteria [1]. Cefpodoxime was quite stable to hydrolysis by β-lactamases produced from B. cereus and E. coli HB101/pBR322 [2].
In vivo: Male ddY mice were administered orally in a volume of 0.2 mL of 0.5% carboxymethyl cellulose sodium salt. After 7 days, it was shown that cefpodoxime had good efficacy against streptococcus spp. and K. pneumoniae infection in mice [1].
References:
[1]. Sakagawa, E., Otsuki, M., Oh, T., & Nishino, T. In-vitro and in-vivo antibacterial activities of CS-834, a new oral carbapenem. Journal of Antimicrobial Chemotherapy, 1998; 42: 426-437.
[2]. Fukuoka, T., Ohya, S., Utsui, Y., Domon, H., Takenouchi, T., Koga, T., … Kuwahara, S. In vitro and in vivo antibacterial activities of CS-834, a novel oral carbapenem. Antimicrobial Agents and Chemotherapy, 1997; 41(12): 2652–2663.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 427.5 |
| Cas No. | 80210-62-4 |
| Formula | C15H17N5O6S2 |
| Synonyms | R 3763 |
| Solubility | ≥50 mg/mL in DMSO |
| Chemical Name | (6R)-7R-[[(2Z)-2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| SDF | Download SDF |
| Canonical SMILES | O=C(N1[C@]2([H])SCC(COC)=C1C(O)=O)[C@H]2NC(/C(C3=CSC(N)=N3)=N\OC)=O |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 细胞实验 [1]: | |
|
细胞系 |
肺炎链球菌 |
|
溶解方法 |
在DMSO中的溶解度≤10mg/ml。为了获得更高的浓度,可以将离心管在37℃加热10分钟和/或在超声波浴中震荡一段时间。原液可以在-20℃以下储存几个月。 |
|
反应时间 |
23.5mg/kg |
|
应用 |
CS-834对由青霉素敏感性肺炎链球菌引起的肺炎的治疗效果优于头孢地尼,与头孢特仑酯和头孢妥仑酯相当,但不如cefpodoxime proxetil。然而,CS-834对由青霉素耐药的肺炎链球菌引起的肺炎表现出最强的治疗效果。 |
| 动物实验 [1]: | |
|
动物模型 |
雄性ddY小鼠 |
|
剂量 |
口服0.2 mL 0.5%羧甲基纤维素钠盐 |
|
应用 |
七天后,口服cefpodoxime的小鼠具有良好的抗链球菌和肺炎克雷伯菌感染的能力。 |
|
注意事项 |
请测试所有化合物在室内的溶解度,实际溶解度和理论值可能略有不同,这是由实验系统的误差引起的,属于正常现象。 |
|
References: [1]. Sakagawa E, Otsuki M, Oh T, Nishino T. In-vitro and in-vivo antibacterial activities of CS-834, a new oral carbapenem. J Antimicrob Chemother. 1998 Oct;42(4):427-37. Erratum in: J Antimicrob Chemother 1999 Jan;43(1):169. Ou T [corrected to Oh T]. PubMed PMID: 9818740. |
|
化学结构