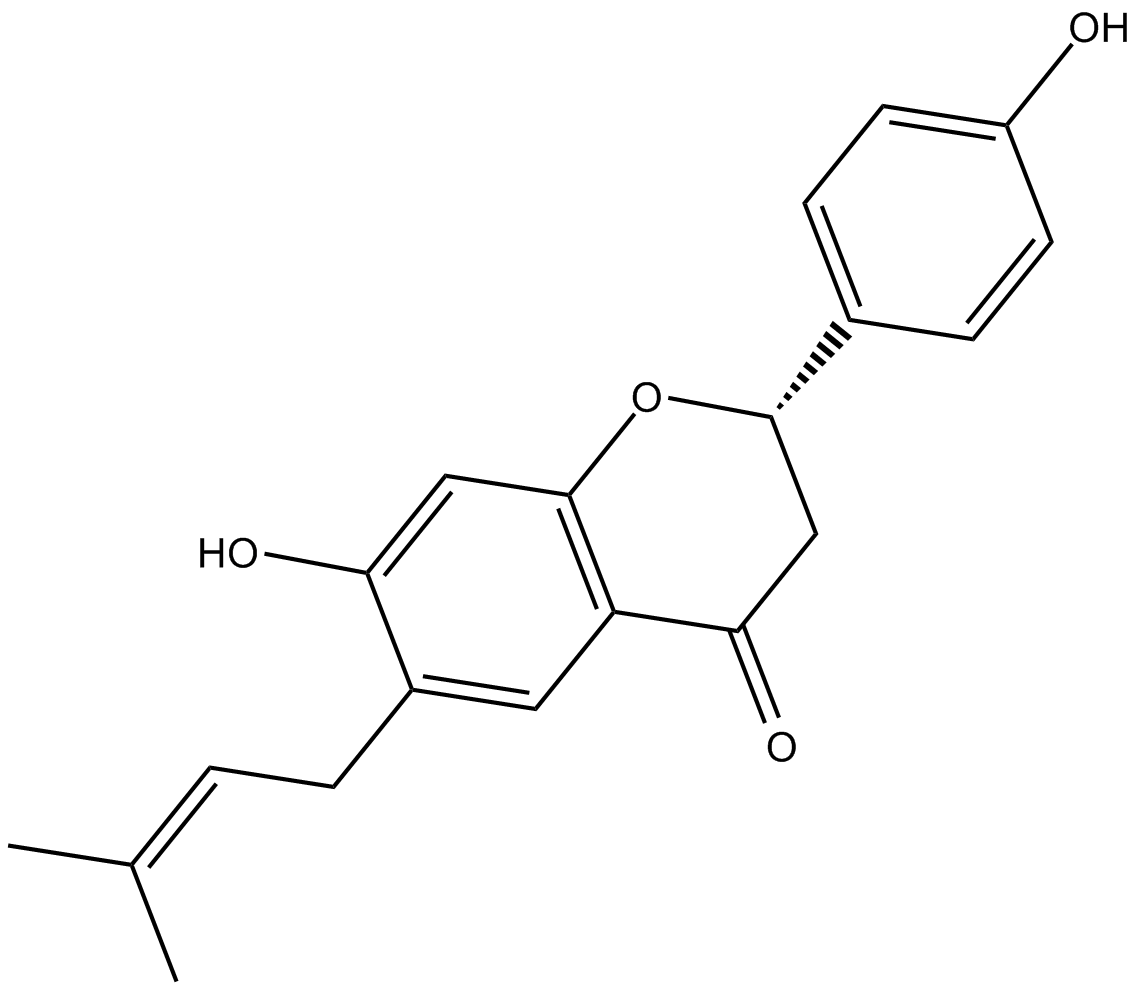
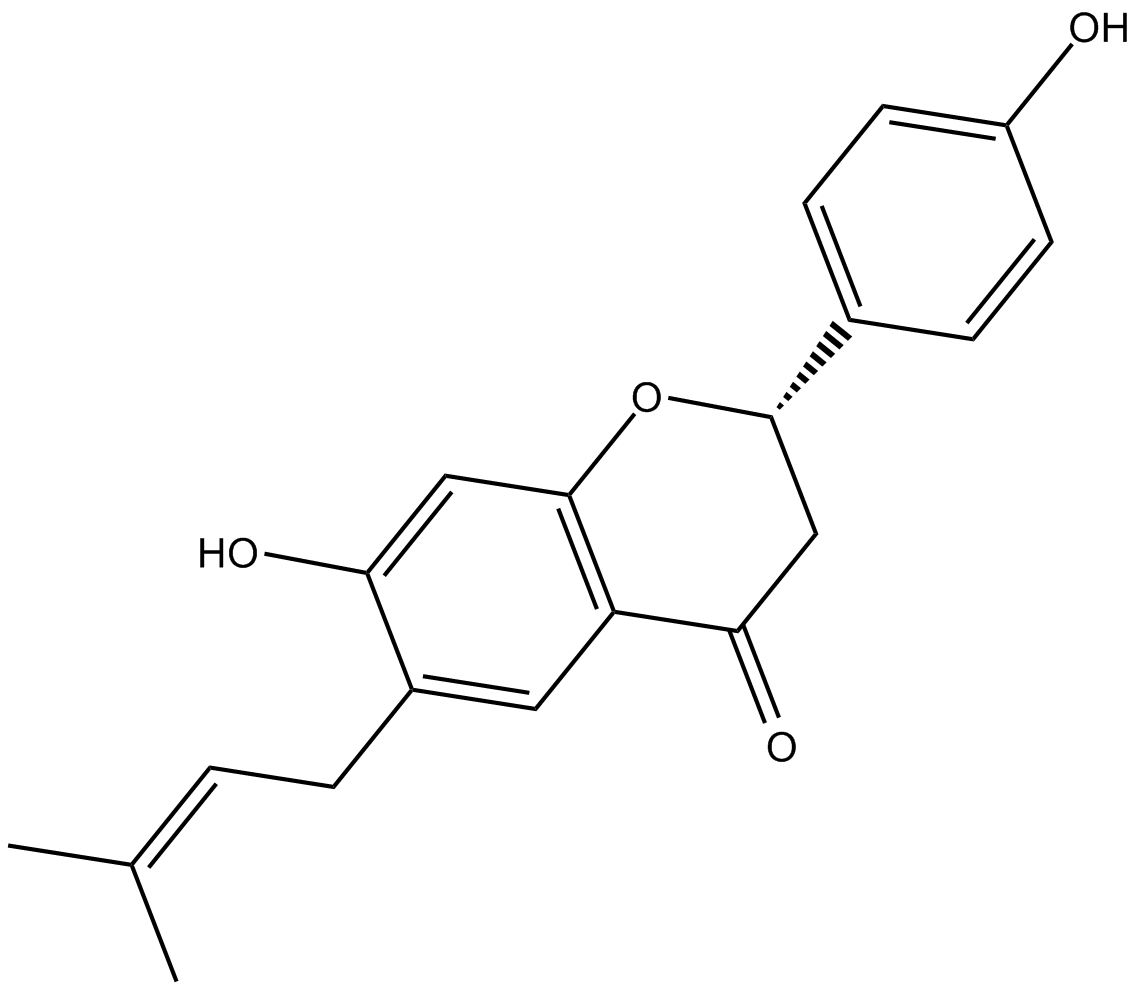
Bavachin
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
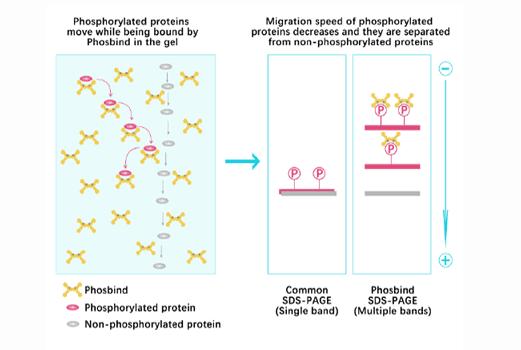
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
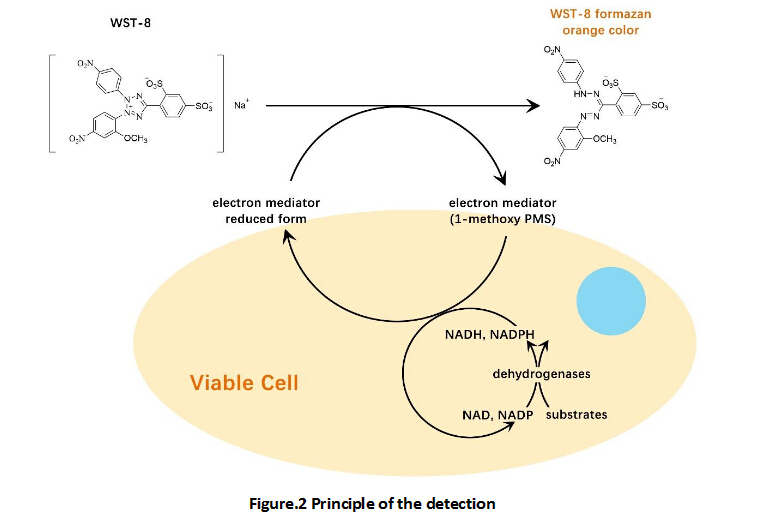
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
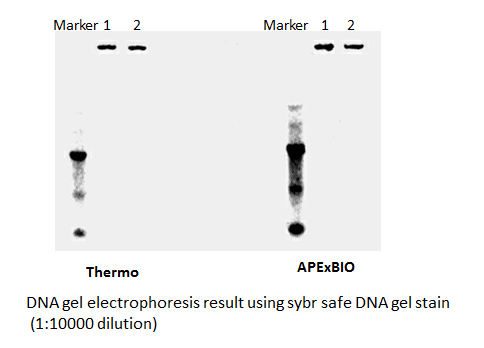
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
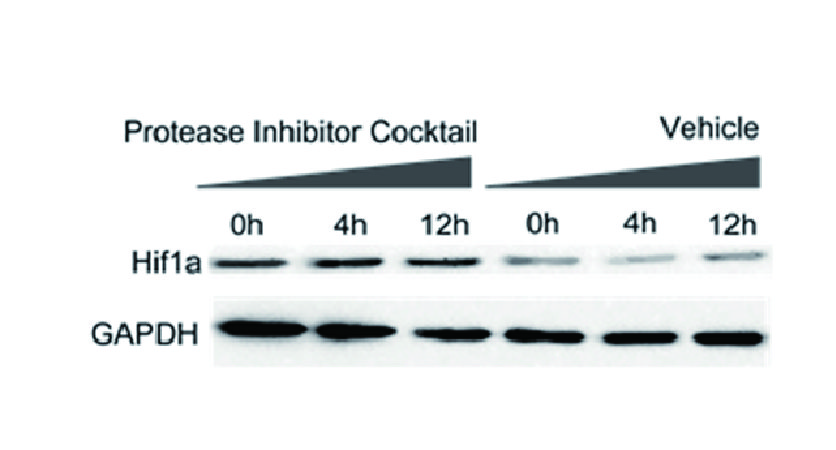
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
Bavachin is an acyl-coenzyme A: cholesterol acyltransferase inhibitor [1].
Acyl-coenzyme A: cholesterol acyl transferase (ACAT) is an enzyme responsible for the intracellular esterification of free cholesterol with fatty acids and plays dominant roles in intestinal absorption of cholesterol, hepatic production of lipoproteins and accumulation of cholesteryl ester within macrophages and smooth muscle cells. [1].
Bavachin showed a significant inhibition of ACAT enzyme. The IC50 value of bavachin was 86.0 μM in the ACAT assay system using rat liver microsome [1]. Bavachin is a flavonoid first isolated from Psoralea corylifolia that has been used as a traditional medicine in Asia. In CV-1 cells transfected with plasmids ERα or ERβ, bavachin showed ER ligand binding activity with an EC50 of 320 nM and 680 nM, respectively. Bavachin increased the mRNA levels of estrogen-responsive genes such as pS2 and PR, and decreased the protein level of ERα by proteasomal pathway [2]. Bavachin activated gene expression of proliferator-activated receptorγ (PPARγ), adipogenic transcriptional factors, and CCAAT/enhancer binding protein-α (C/EBPα). Bavachin increased adiponectin expression and secretion in adipocytes. Bavachin increased insulin-induced glucose uptake by differentiated adipocytes and myoblasts. In differentiated adipocytes, bavachin enhanced glucose uptake [3].
References:
[1] Choi J H, Rho M C, Lee S W, et al. Bavachin and isobavachalcone, acyl-coenzyme A: cholesterol acyltransferase inhibitors from Psoralea corylifolia[J]. Archives of pharmacal research, 2008, 31(11): 1419-1423.
[2] Park J, Kim D H, Ahn H N, et al. Activation of estrogen receptor by bavachin from Psoralea corylifolia[J]. Biomolecules & therapeutics, 2012, 20(2): 183-188.
[3] Lee H, Li H, Noh M, et al. Bavachin from Psoralea corylifolia improves insulin-dependent glucose uptake through insulin signaling and AMPK activation in 3T3-L1 adipocytes[J]. International journal of molecular sciences, 2016, 17(4): 527.
| Physical Appearance | A crystalline solid |
| Storage | Store at -20°C |
| M.Wt | 324.4 |
| Cas No. | 19879-32-4 |
| Formula | C20H20O4 |
| Synonyms | Corylifolin |
| Solubility | ≤20mg/ml in ethanol;30mg/ml in DMSO;50mg/ml in dimethyl formamide |
| Chemical Name | (2S)-2,3-dihydro-7-hydroxy-2-(4-hydroxyphenyl)-6-(3-methyl-2-butenyl)-4H-1-benzopyran-4-one |
| SDF | Download SDF |
| Canonical SMILES | OC1=CC(O[C@H](C2=CC=C(O)C=C2)CC3=O)=C3C=C1C/C=C(C)/C |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |