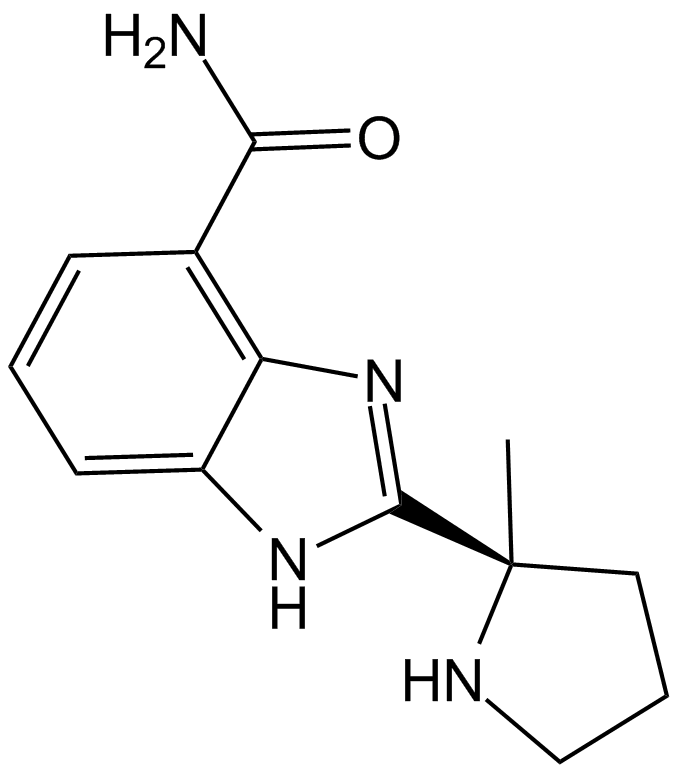
ABT-888 (Veliparib)
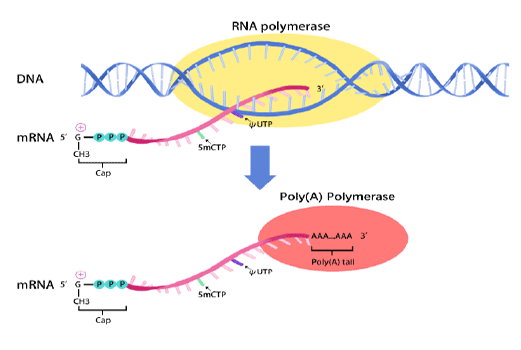
mRNA synthesis
In vitro transcription of capped mRNA with modified nucleotides and Poly(A) tail
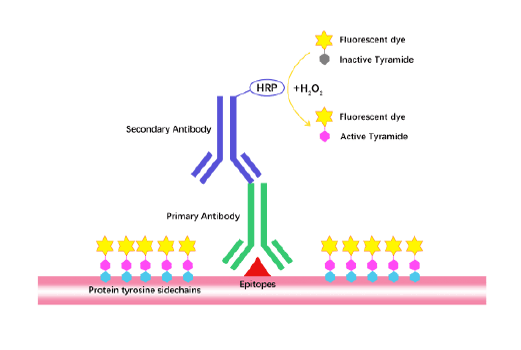
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
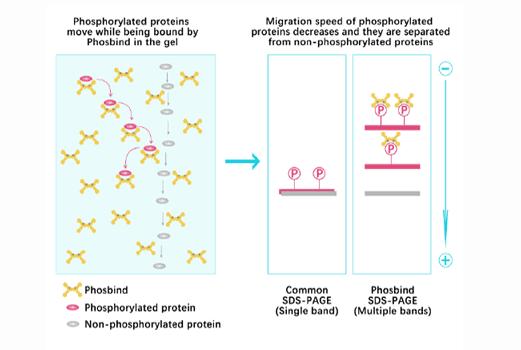
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
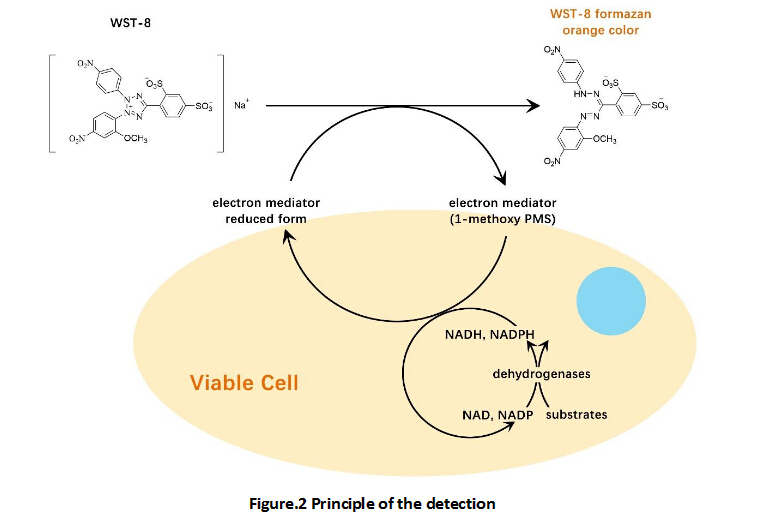
Cell Counting Kit-8 (CCK-8)
A convenient and sensitive way for cell proliferation assay and cytotoxicity assay
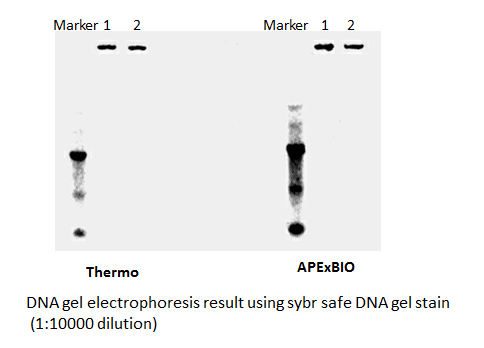
SYBR Safe DNA Gel Stain
Safe and sensitive stain for visualization of DNA or RNA in agarose or acrylamide gels.
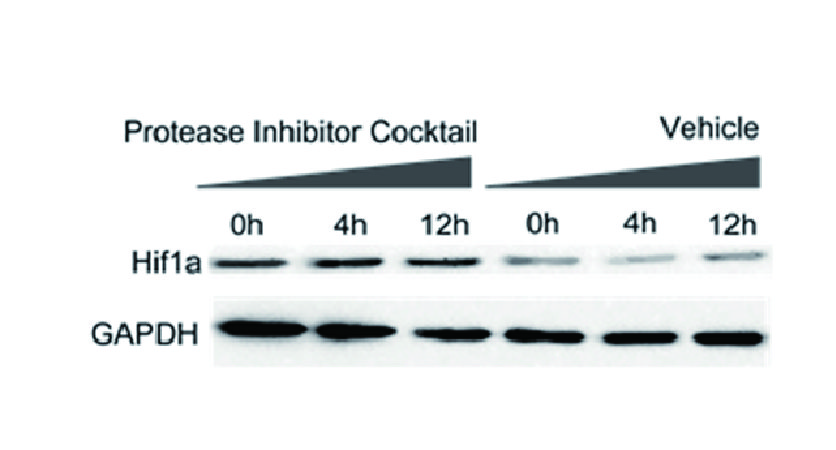
Inhibitor Cocktails
Protect the integrity of proteins from multiple proteases and phosphatases for different applications.
ABT-888 (Veliparib)是聚(ADP-核糖)聚合酶(PARP)的抑制剂。在临床前肿瘤模型中,ABT-888 (Veliparib)与各种细胞毒性药物联合使用显示了良好的体内功效。PARP参与DNA修复。PARP水平的升高会导致对细胞毒性化疗和放疗的抵抗性。因此,PARP抑制剂有望用于化疗和放疗的增敏剂。与微卫星稳定(MSS)的细胞系相比,ABT-888在MRE11和RAD50基因突变的微卫星不稳定性(MSI)细胞系中也是有活性的。
参考文献:
1. Shivaani Kummar, Robert Kinders, Martin E. Gutierrez, Larry Rubinstein, Ralph E. Parchment, Lawrence R. Phillips, Jiuping Ji, Anne Monks, Jennifer A. Low, Alice Chen, Anthony J. Murgo, Jerry Collins, Seth M. Steinberg, Helen Eliopoulos, Vincent L. Giranda, Gary Gordon, Lee Helman, Robert Wiltrout, Joseph E. Tomaszewski and James H. Doroshow. Phase 0 Clinical Trial of the Poly (ADP-Ribose) Polymerase Inhibitor ABT-888 in Patients With Advanced Malignancies. Journal of Clinical Oncology. 2009; 27(16): 2705 – 11.
2. Xiaofeng Li, Juergen Delzer, Richard Voorman, Sonia M. de Morais and Yanbin Lao. Disposition and Drug-Drug Interaction Potential of Veliparib (ABT-888), a Novel and Potent Inhibitor of Poly (ADP-ribose) Polymerase. DRUG METABOLISM AND DISPOSITION. 2011; 39(7): 1161 – 69.
3. E. Vilar Sanchez, A. Chow, L. Raskin, M. D. Iniesta, B. Mukherjee and S. B. Gruber. Preclinical testing of the PARP inhibitor ABT-888 in microsatellite instable colorectal cancer. Journal of Clinical Oncology. 2009; 27(15S): 11028A.
- 1. Masato Mashimo, Momoko Kita, et al. "Tankyrase Regulates Neurite Outgrowth through Poly (ADP-ribosyl) ation-Dependent Activation of β-Catenin Signaling." Int J Mol Sci. 2022 Mar 4;23(5):2834. PMID: 35269974
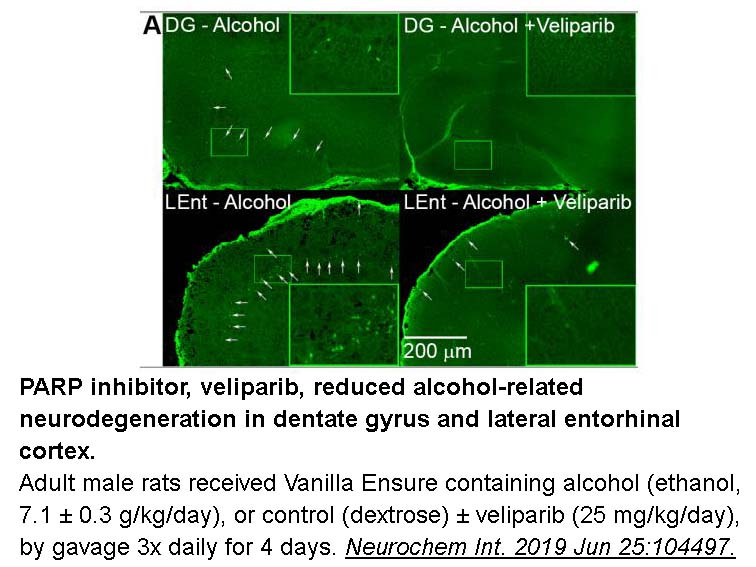
- 2. Kouzoukas DE, Schreiber JA, et al. "PARP inhibition blocks alcohol-induced neurodegeneration and neuroinflammatory cytosolic phospholipase A2 elevations." Neurochem Int. 2019 Jun 25:104497. PMID: 31251945
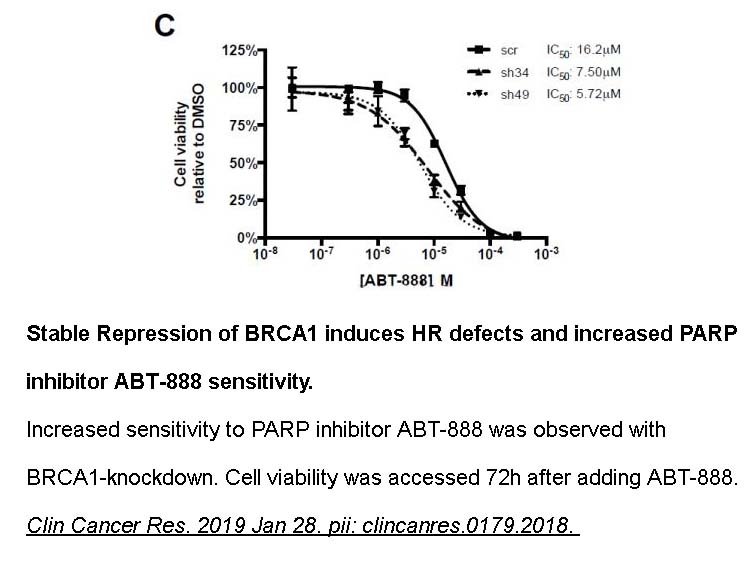
- 3. Poh W, Dilley RL, et al. "BRCA1 Promoter Methylation Is Linked to Defective Homologous Recombination Repair and Elevated miR-155 to Disrupt Myeloid Differentiation in Myeloidb Malignancies." Clin Cancer Res. 2019 Jan 28. PMID: 30692098
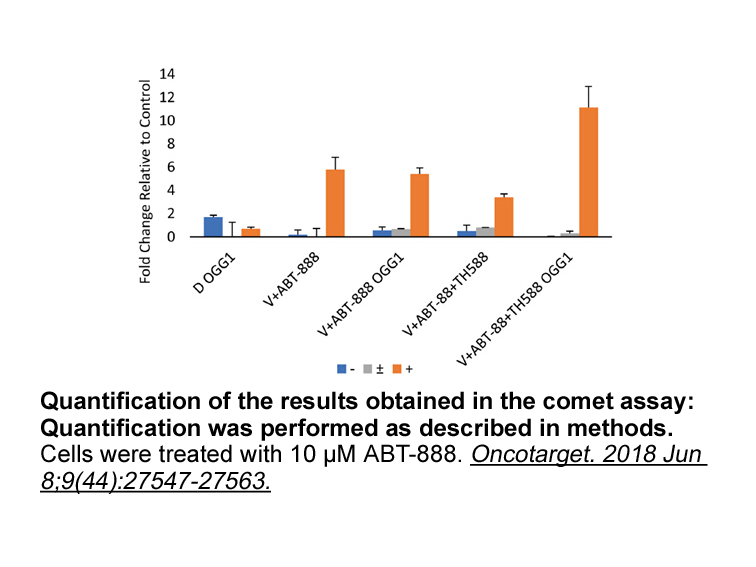
- 4. Versano Z, Shany E, et al. "MutT homolog 1 counteracts the effect of anti-neoplastic treatments in adult and pediatric glioblastoma cells." Oncotarget. 2018 Jun 8;9(44):27547-27563. PMID: 29938005
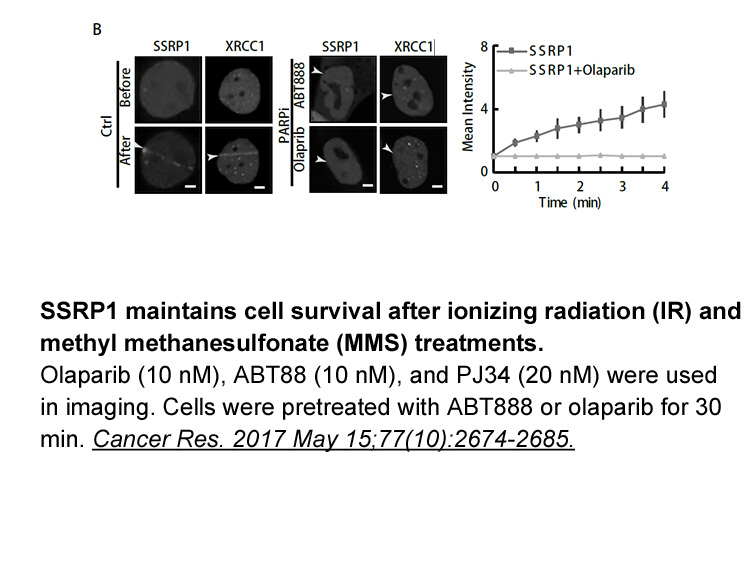
- 5. Gao Y, Li C, et al. "SSRP1 Cooperates with PARP and XRCC1 to Facilitate Single-Strand DNA Break Repair by Chromatin Priming." Cancer Res. 2017 May 15;77(10):2674-2685. PMID: 28416484
- 6. Wang X, Sekine Y, et al."Inhibition of Poly-ADP-Ribosylation Fails to Increase Axonal Regeneration or Improve Functional Recovery after Adult Mammalian CNS Injury." eNeuro. 2016 Dec 26;3(6). PMID: 28032120
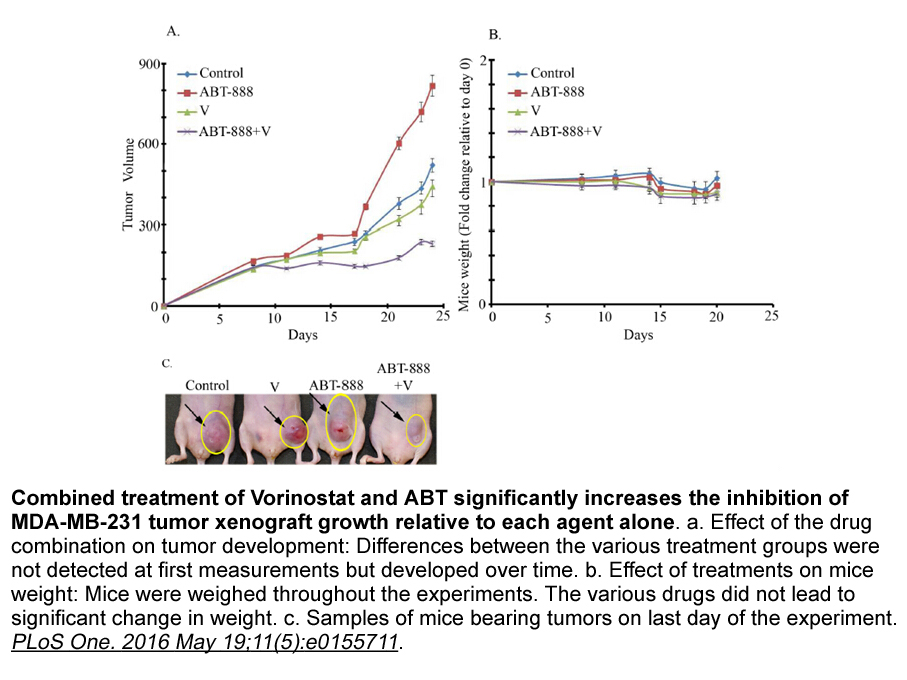
- 7. Yalon M, Tuval-Kochen L, et al. "Overcoming Resistance of Cancer Cells to PARP-1 Inhibitors with Three Different Drug Combinations." PLoS One. 2016 May 19;11(5):e0155711. PMID: 27196668
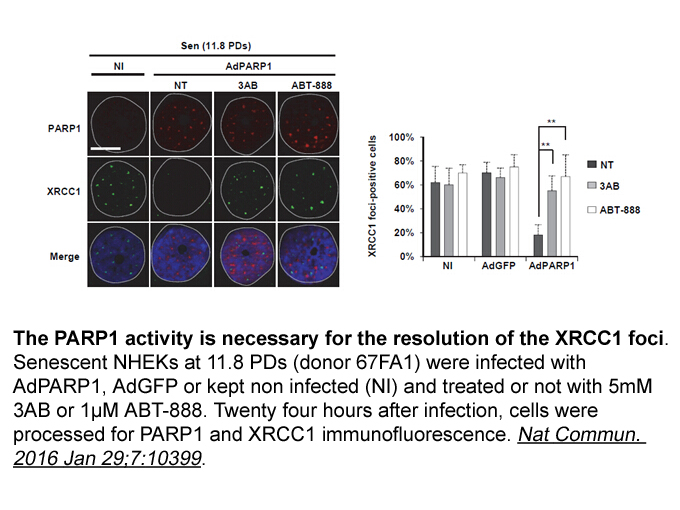
- 8. Nassour J, Martien S, et al."Defective DNA single-strand break repair is responsible for senescence and neoplastic escape of epithelial cells." Nat Commun. 2016 Jan 29;7:10399. PMID: 26822533
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 244.3 |
| Cas No. | 912444-00-9 |
| Formula | C13H16N4O |
| Synonyms | ABT-888,ABT 888,ABT888,Veliparib |
| Solubility | insoluble in H2O; ≥10.6 mg/mL in EtOH with ultrasonic; ≥6.11 mg/mL in DMSO |
| Chemical Name | 1-[3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one |
| SDF | Download SDF |
| Canonical SMILES | CC1(CCCN1)C2=NC3=C(C=CC=C3N2)C(=O)N |
| 运输条件 | 蓝冰运输或根据您的需求运输。 |
| 一般建议 | 不同厂家不同批次产品溶解度各有差异,仅做参考。若实验所需浓度过大至产品溶解极限,请添加助溶剂助溶或自行调整浓度。溶液形式一般不宜长期储存,请尽快用完。 |
| 细胞实验: | |
|
细胞系 |
HCT-116和HT-29细胞系 |
|
溶解方法 |
在DMSO中的溶解度>10 mM。为了获得更高的浓度,可以将离心管在37℃加热10分钟和/或在超声波浴中震荡一段时间。原液可以在-20℃以下储存几个月。 |
|
反应条件 |
4 μM;24 h |
|
应用 |
在HCT-116和HT-29细胞系中,用SRB实验检测ABT-888对抗癌剂SN38或oxaliplatin的协同作用。在SN38与ABT-888联合处理的样品中,PARP的活性显著减少(>4倍;24 h)。 |
| 动物实验: | |
|
动物模型 |
雌性无胸腺裸鼠 |
|
剂量 |
12.5 mg/kg;口服给药,2次/天,间隔6小时。 |
|
应用 |
在5-6周龄的雌性无胸腺裸鼠中,在每侧的皮下注射200 mL细胞悬浮液(5*106细胞)建立HCT116异种移植物。与RT和CPT-11,而非ABT-888治疗的肿瘤相比,三重疗法组(RT、CPT-11和ABT)具有显著更长的肿瘤生长延迟(TGD),其平均TGD为14.21天。 |
|
注意事项 |
请测试所有化合物在室内的溶解度,实际溶解度和理论值可能略有不同。这是由实验系统的误差引起的,属于正常现象。 |
|
References: [1] Davidson D, Wang Y, Aloyz R, et al. The PARP inhibitor ABT-888 synergizes irinotecan treatment of colon cancer cell lines[J]. Investigational new drugs, 2013, 31(2): 461-468. [2] Shelton J W, Waxweiler T V, Landry J, et al. In vitro and in vivo enhancement of chemoradiation using the oral parp inhibitor ABT-888 in colorectal cancer cells[J]. International Journal of Radiation Oncology* Biology* Physics, 2013, 86(3): 469-476. |
|
| Veliparib (ABT-888)是一个有效的PARP1和PARP2抑制剂,Ki值分别为5.2 nM和2.9 nM。. | ||||||
| 靶点 | PARP1 | PARP2 | ||||
| IC50 | 5.2 nM (Ki) | 2.9 nM (Ki) | ||||
质量控制和MSDS
- 批次:
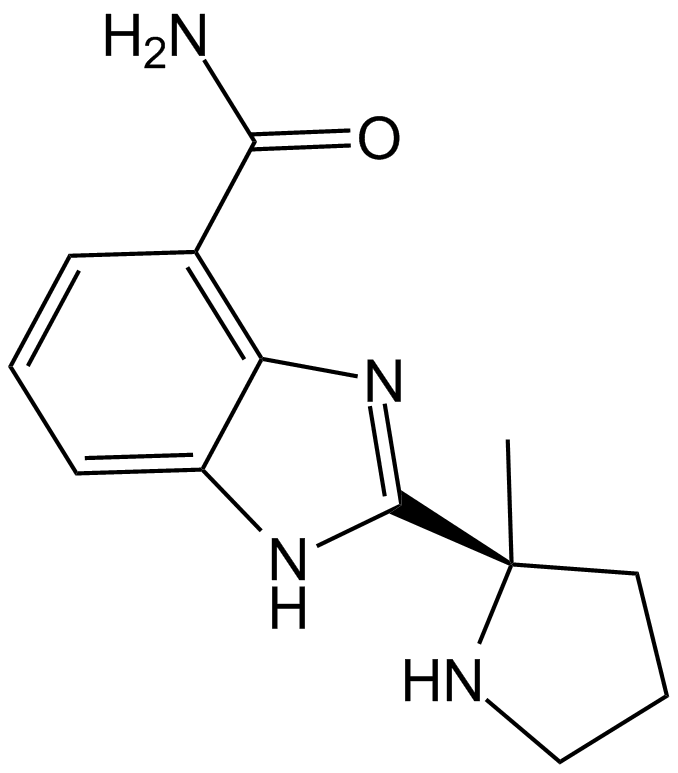
化学结构
相关生物数据
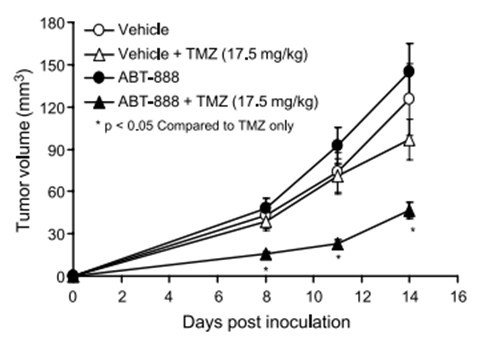
相关生物数据
相关生物数据
相关生物数据
相关生物数据
相关生物数据
相关生物数据